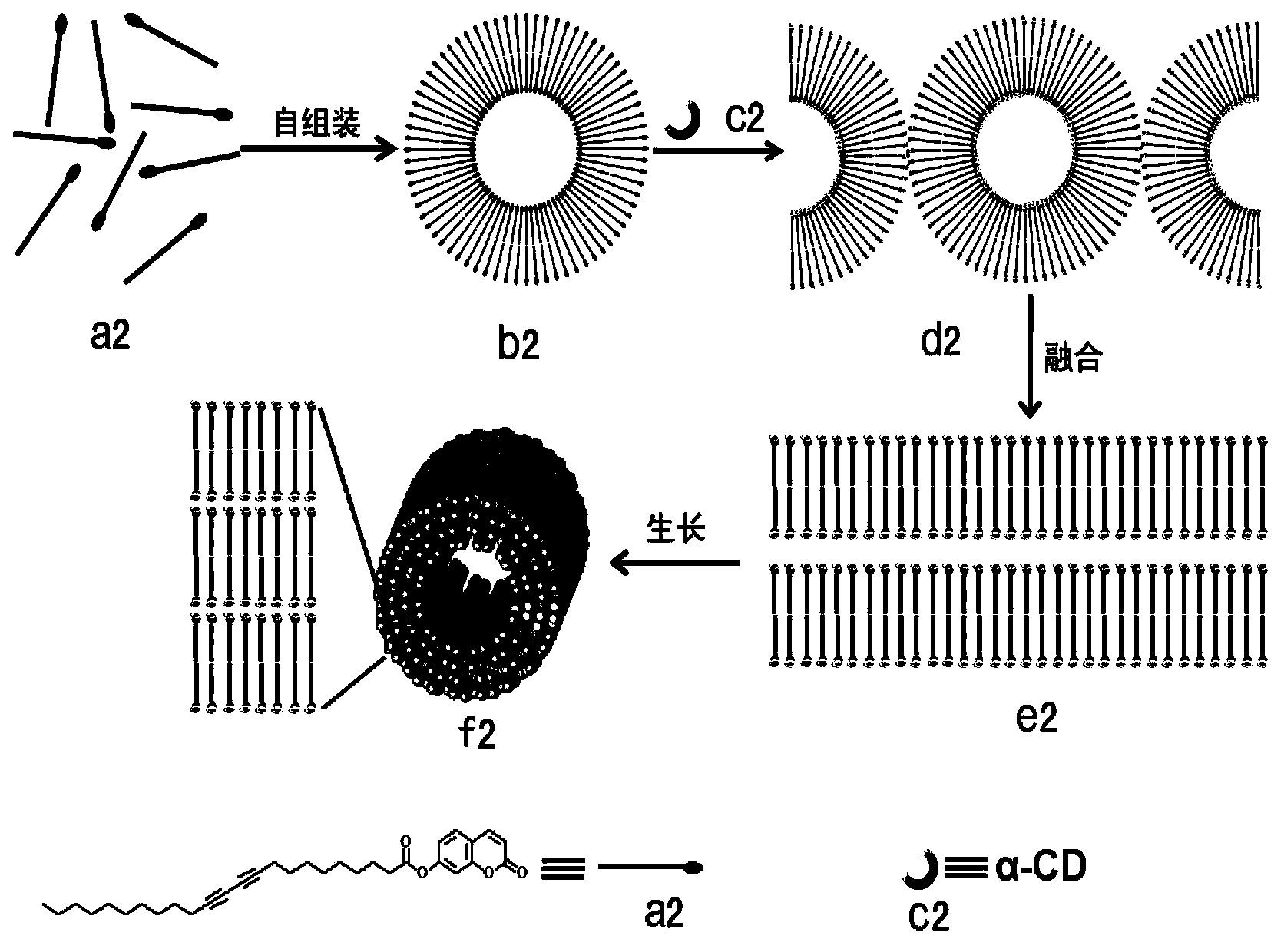
Polydiacetylene micro-tube material and preparation method thereof by using hierarchical self-assembly
A technology of microtubes and diacetylene, which is applied in the field of graded self-assembly to prepare polydiacetylene microtube materials, can solve problems affecting performance and practical application, harsh self-assembly conditions, poor operability, etc., to overcome system operation and practical application, The effect of realizing hierarchical self-assembly and simplifying self-assembly conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
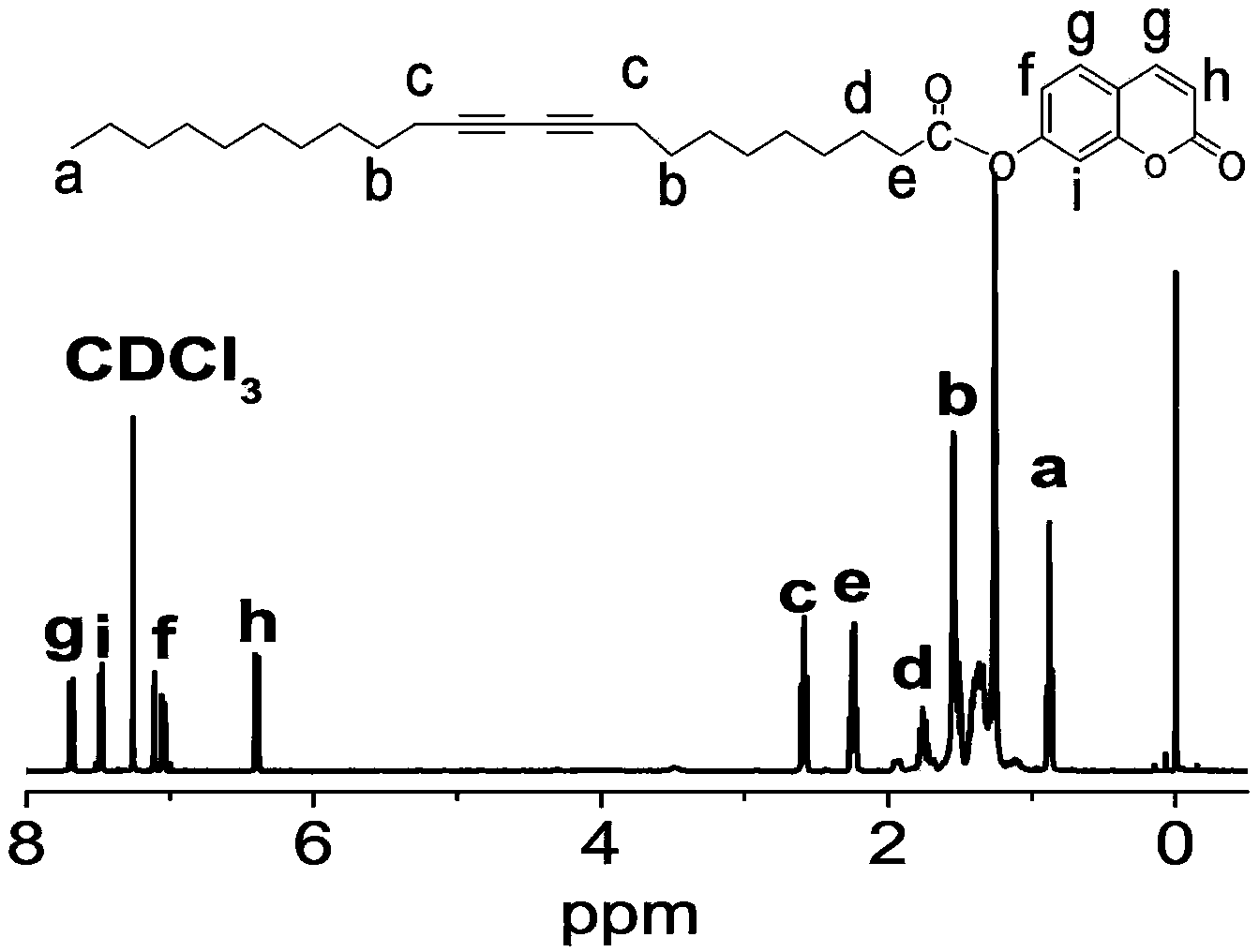
[0037] First weigh 52.0mg of 23-diynoic acid, 24.0mg of 7-hydroxycoumarin, 39.5mg of N,N'-dicyclohexylcarbodiimide and 3.0mg of 4-dimethylaminopyridine, dissolve in 35ml of refined dichloromethane , stirred at 25°C for 72 hours, filtered, and the filtrate was washed successively with 30ml of water, 1 mol / liter of hydrochloric acid, 6% aqueous sodium bicarbonate solution and water, and dried the dichloromethane solution with magnesium sulfate. The solvent dichloromethane was spun off to obtain a crude product of coumarindiyne, which was purified by column chromatography with a yield of 82%.
[0038] Weigh 4.9 mg of coumarin diyne monomer, dissolve it in 1.2 ml of ethanol under ultrasound to form a uniform and transparent solution, then introduce the solution into 200 ml of deionized water at one time, and then place it in a water bath at 75°C to keep the After the temperature was sonicated for 80 minutes, it was cooled to room temperature in the dark, and stored in a refrigerat...
Embodiment 2
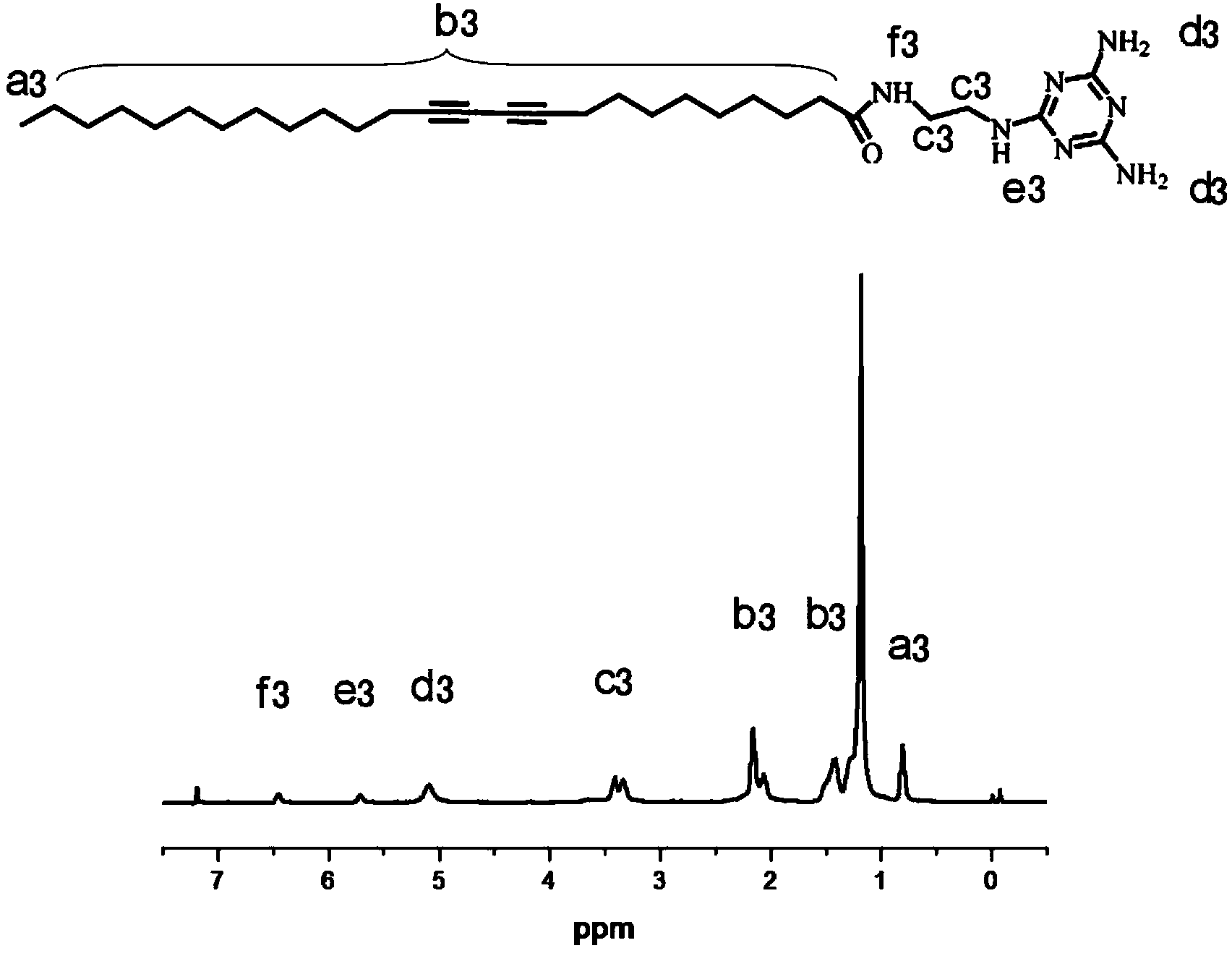
[0045] Weigh 56.0mg of pentacicodiynoic acid, dissolve in 25ml of refined di Methylene chloride was reacted for half an hour, the solvent dichloromethane was removed by rotary evaporation, the resultant was dissolved in 30ml of anhydrous ether, filtered, the filtrate was extracted three times with distilled water, and the organic phase was spin-dried to obtain an intermediate product. The intermediate product was dissolved in 25 ml of refined dichloromethane, 27.0 mg of refined ethylenediamine was added, and the reaction was stirred at room temperature for 2 hours to obtain the crude product ethylenediaminediyne. The crude product was purified by column chromatography with a yield of 90%.
[0046] Weigh 16.8mg of ethylenediaminediyne and dissolve it in 20ml of refined 1,4-dioxane, add 6.0mg of 2-chloro-4,6-diamino-1,3,5-triazine, in anaerobic Heating and reacting at 99°C for 24 hours under the conditions. After the reaction was completed, the solvent was removed by rotary eva...
Embodiment 3
[0054] Weigh 49.0mg of 23-diynoic acid, 28.0mg of 7-hydroxycoumarin, 38.5mg of N,N'-dicyclohexylcarbodiimide and 2.5mg of 4-dimethylaminopyridine, dissolve in 30ml of refined dichloromethane, Stir at 25°C for 72 hours, filter, and wash the filtrate successively with 25ml of water, 1 mol / liter of hydrochloric acid, 6% sodium bicarbonate aqueous solution and water, then dry the dichloromethane solution with magnesium sulfate, spin The dichloromethane was removed from the solvent to obtain a crude product of coumarindiyne, which was purified by column chromatography with a yield of 86%.
[0055] Weigh 9.8 mg of coumarin diyne monomer, dissolve it in 1.5 ml of ethanol under ultrasound to form a uniform and transparent solution; introduce the solution into 500 ml of deionized water at one time, and then place it in a water bath at 75°C to maintain the temperature After ultrasonication for 75 minutes, it was cooled to room temperature in the dark, and then stored in a refrigerator a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com