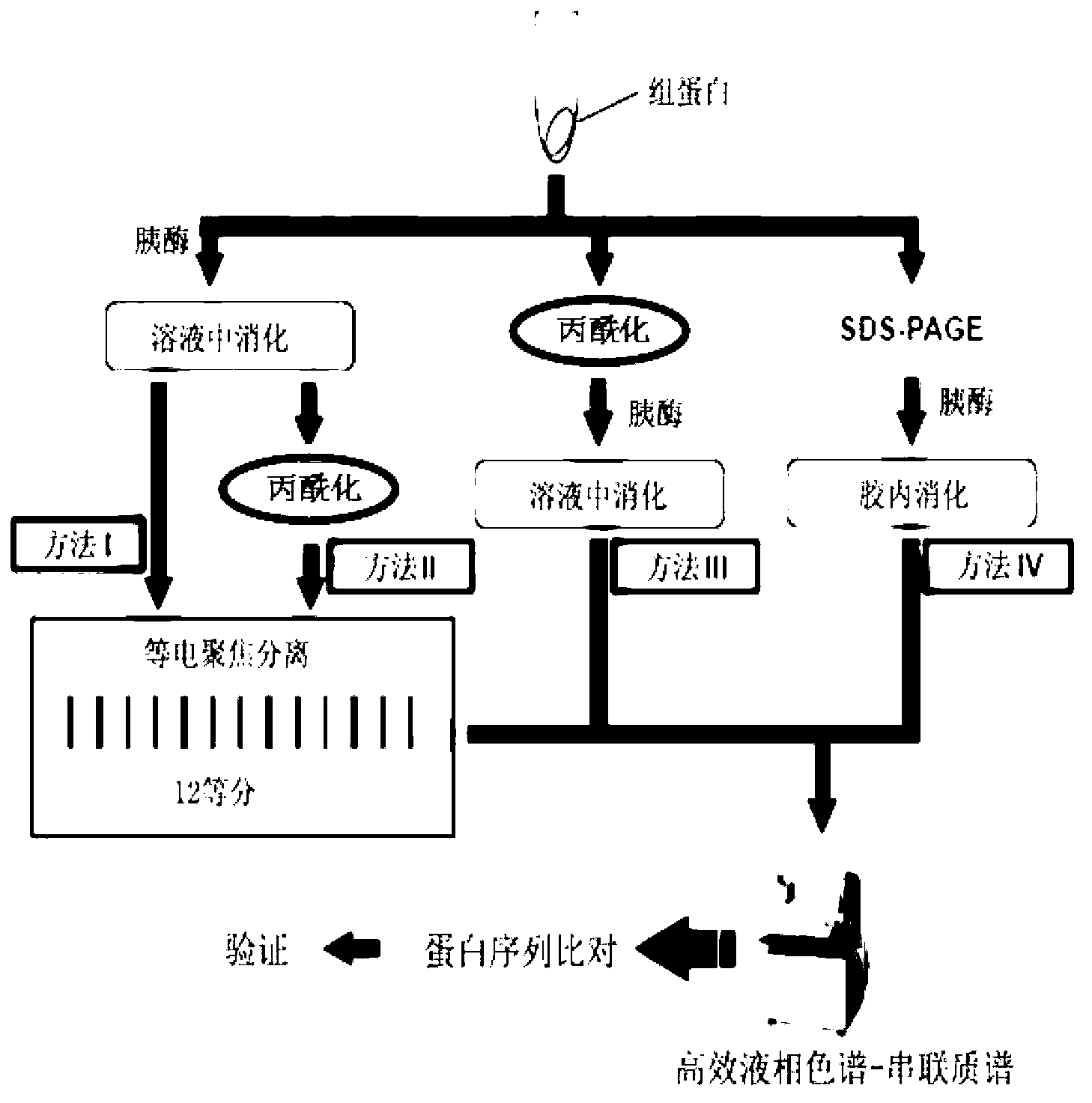
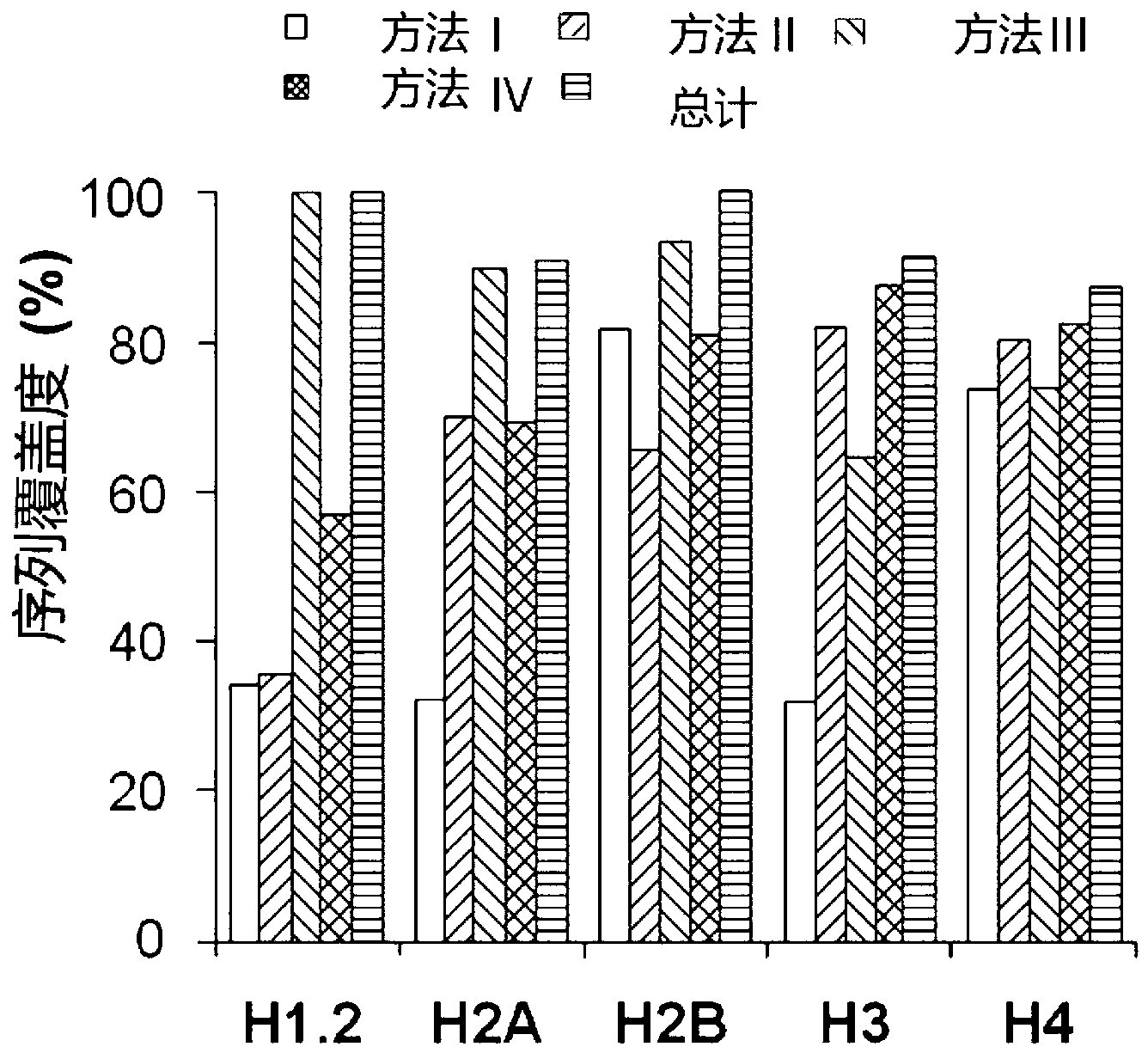
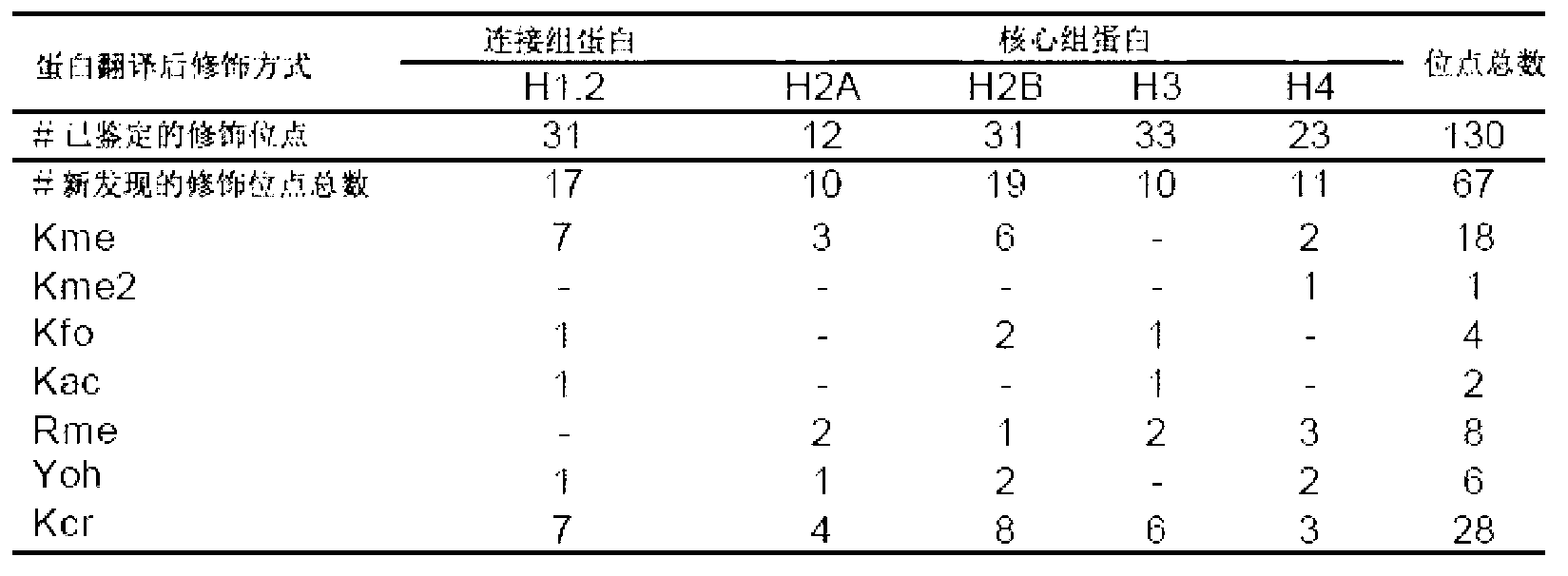
A method of detecting protein lysine crotonylation acylation modification and developing affinity reagents
A technique for crotonylation and histone, applied in the field of reagents for detecting protein crotonylation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0118] All polypeptides of the present invention are entrusted to a synthetic company to synthesize, and Fmoc-Lysine (crotonylated tonyl)-OH is used to protect amino acids. All chemical reagents and Flag M2 antibody were purchased from Sigma-Aldrich (St. Louis, MO). All chemicals were of the highest purity or analytical grade commercially available. HA antibody was purchased from Roche Diagnostics (Indianapolis, IN). Histones were extracted using previously reported methods (see Shechter, D., Dormann, H.L., Allis, C.D., and Hake, S.B. (2007). Extraction, purification and analysis of histones. Nat Protoc 2, 1445-1457.; Tateishi , K., Okada, Y., Kallin, E.M., and Zhang, Y. (2009). Role of Jhdm2a in regulating metabolic gene expression and obesity resistance. Nature 458, 757-76 1.) From S. cerevisiae S cells, S2 cells, mouse embryonic fibroblast (MEF) cells, Caucasian human embryonic lung fibroblasts (IMR90) and human HeLa cells. 4,4,4,3-D4-Crotonic acid was synthesized from ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap