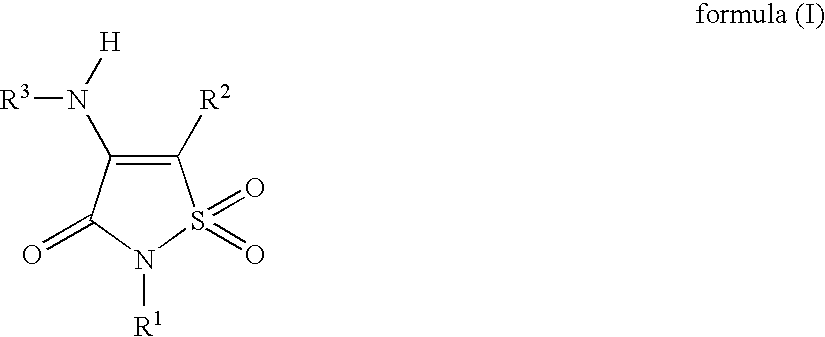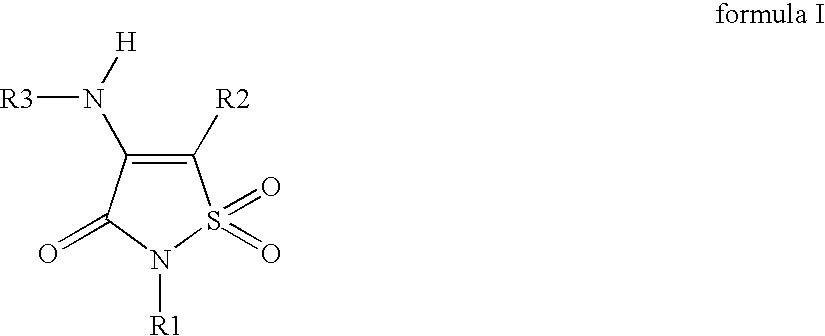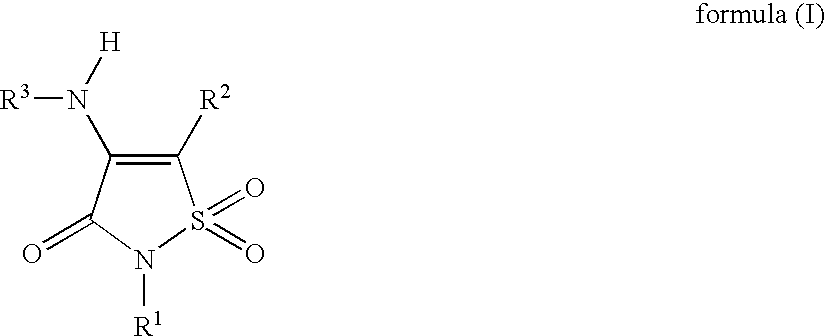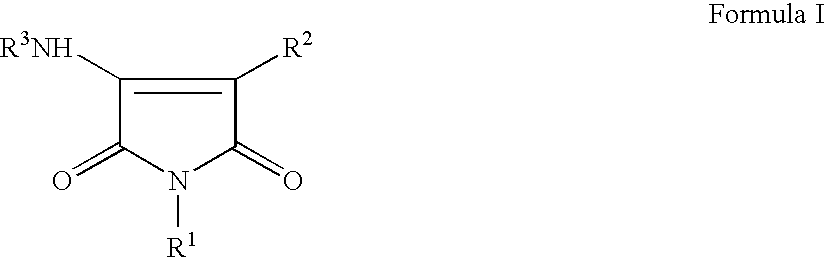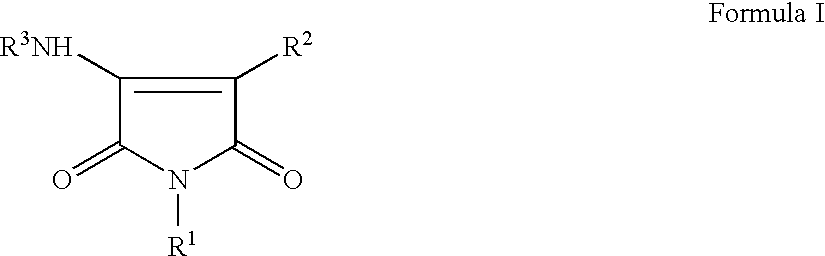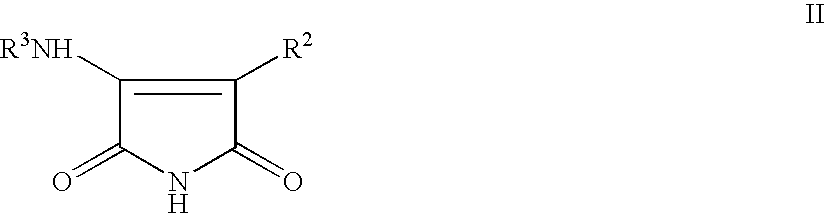Patents
Literature
74 results about "Liver X receptor" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
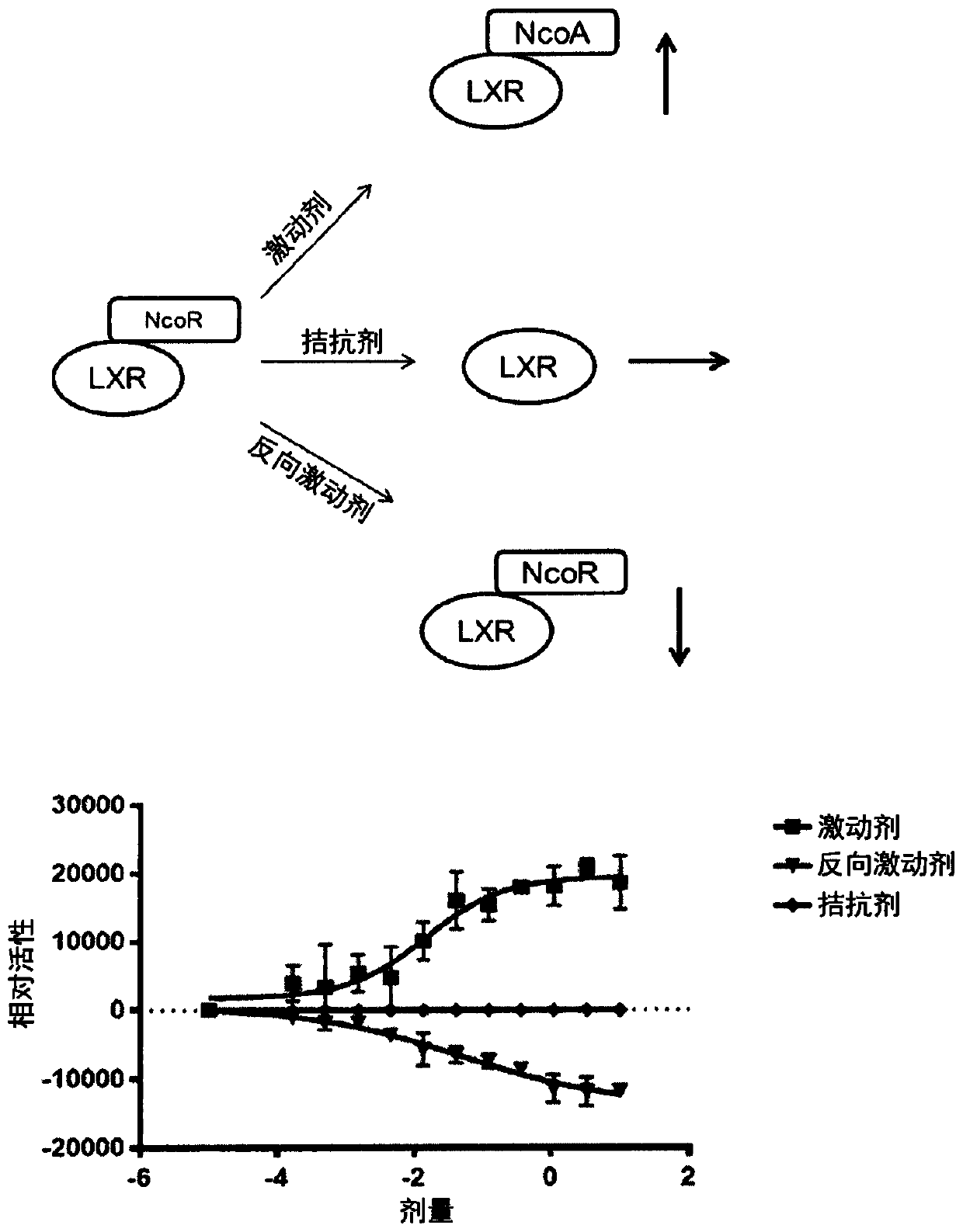
The liver X receptor (LXR) is a member of the nuclear receptor family of transcription factors and is closely related to nuclear receptors such as the PPARs, FXR and RXR. Liver X receptors (LXRs) are important regulators of cholesterol, fatty acid, and glucose homeostasis. LXRs were earlier classified as orphan nuclear receptors, however, upon discovery of endogenous oxysterols as ligands, they were subsequently deorphanized.
Method for preparing retinal pigment epithelia
InactiveCN102618488AAvoid potential risksHigh differentiation efficiencyVertebrate cellsArtificial cell constructsAnimal SourcesBiology
The invention discloses a method for preparing retinal pigment epithelia. According to the method, stem cells are induced by utilizing a liver X receptor (LXR) activating agent to be differentiated into the retinal pigment epithelia. Compared with the prior art, the method for inducing the stem cells to be differentiated into the retinal pigment epithelia by utilizing the LXR receptor activating agent has the advantages that: a feed layer is not used, so that potential risks caused by an animal source feed layer are avoided; and the differentiation efficiency can be improved, and the differentiation time can be shortened.
Owner:THE FIRST AFFILIATED HOSPITAL OF THIRD MILITARY MEDICAL UNIVERSITY OF PLA
5-Lipoxygenase modulators
The present invention provides the use of Liver X Receptor (LXR) modulators that have been identified to downregulate 5-lipoxygenase gene expression in order to treat various diseases and disorders that involve the function of the 5-LO protein in intracellular signaling (or other cellular processes) or the function of protein products downstream of 5-LO in intracellular signaling (i.e., leukotrienes).
Owner:WYETH LLC
Pyrrole-2,5-dithione derivatives as liver x receptor modulators
The present invention relates to certain novel compounds of the Formula I to processes for preparing such compounds, to their the utility in modulation of nuclear hormone receptors Liver X Receptor (LXR) α (NR1H3) and / or β (NR1H2) and in treating clinical conditions including cardiovascular diseases such as atherosclerosis; inflammatory diseases, Alzheimer's disease, lipid disorders (dyslipidemias) whether or not associated with insulin resistance, type 2 diabetes and other manifestations of the metabolic syndrome, to methods for their therapeutic use and to pharmaceutical compositions containing them.
Owner:ASTRAZENECA AB
Compounds as liver X receptor modifier
The invention discloses compounds as a liver X receptor modifier, and in particular relates to resveratrol tetramer compounds. The compounds can be used for preparing a medicament for treating or preventing diseases or symptoms related to the activity of a liver X receptor (LXR), and the medicament can prevent and treat neurodegenerative disease, cardiovascular disease, diabetes, hypercholesterolemia or obesity.
Owner:INST OF MATERIA MEDICA CHINESE ACAD OF MEDICAL SCI
Indolyl derivatives as liver-X-receptor (LXR) modulators
Owner:F HOFFMANN LA ROCHE & CO AG
Application of saringosterol
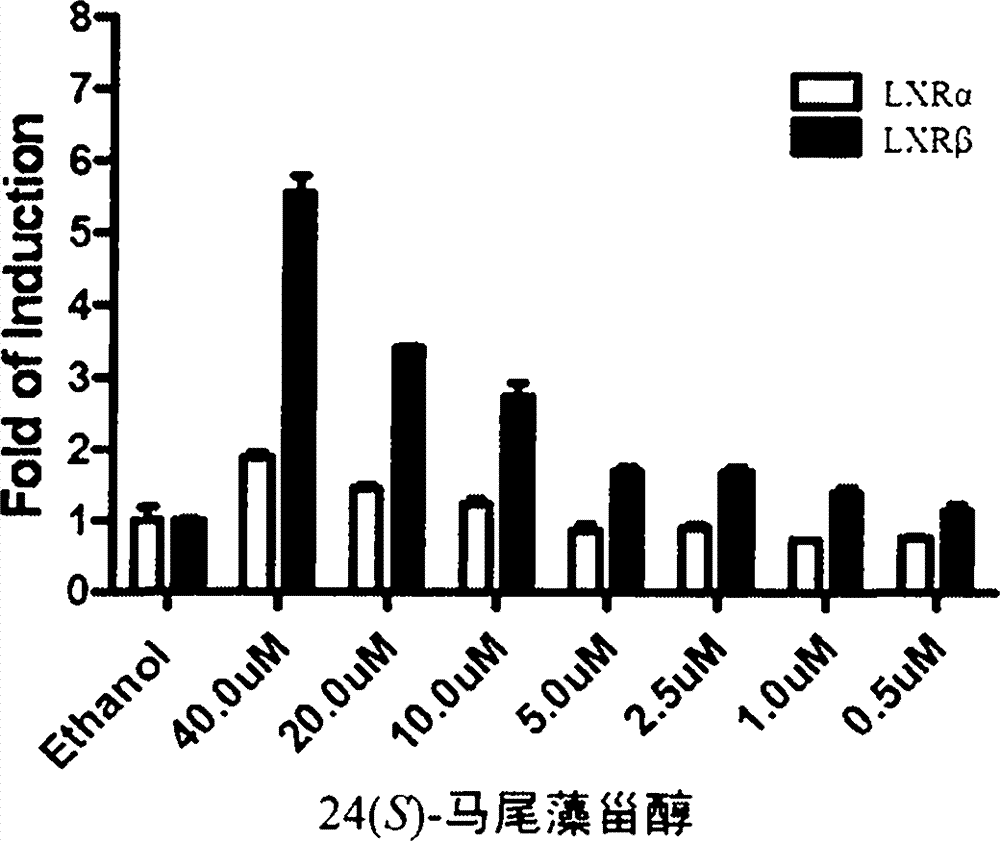
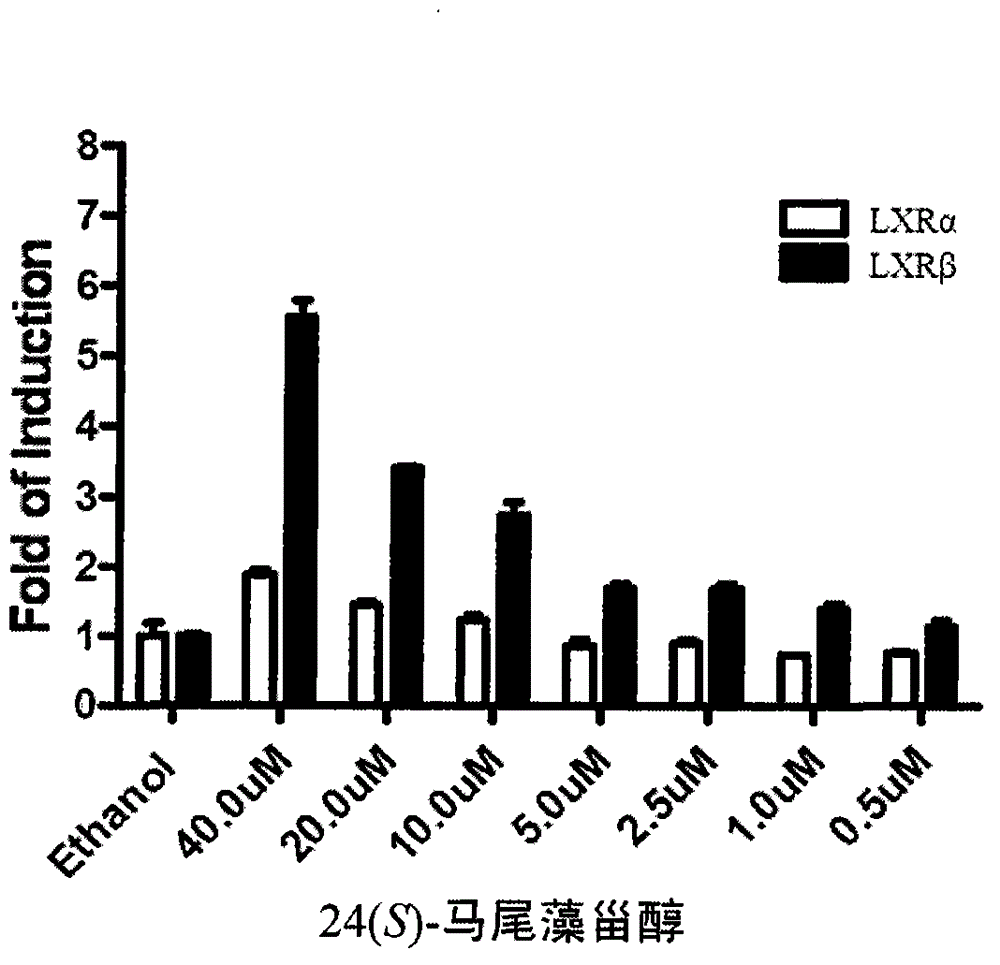
The invention discloses an application of saringosterol to preparation of an LXR (liver X receptor) excitant, and particularly discloses an application of 24(S)-saringosterol as an LXR beta excitant.
Owner:OCEAN UNIV OF CHINA
Extracts with liver-X-receptor modulators, compounds and their use especially in weight control
The invention relates to the use, or methods (especially with regard to animals, especially human, that are in need of such treatment) comprising the use, of an extract and / or one or more natural compounds from plants or parts of plants, respectively, from a genus selected from the group consisting of Schisandra,Illicium, Kadsura, Steganotaenia and Magnolia, alone or as supplement, as active ingredient in the regulation of body weight and / or fat loss and / or for the management of obesity, either in humans or in animals, to the use of said extract and / or natural compound(s) or mixtures in the manufacture of a pharmaceutical or nutraceutical formulation for the regulation of body weight and / or fat loss and / or for the management of obesity either in humans or in animals. The above extract and / or compound(s) can further be used to reduce one or more adverse metabolic parameters in a subject. The invention relates also to said extract and / or compound(s) for use in the treatment or in the preparation of a medicament for the treatment of obesity, as well as their preparation. It also relates to pharmaceutical or nutraceutical formulations comprising said extract and / or natural compound(s) which are useful in the regulation of body weight and / or fat loss and / or for the management of obesity.
Owner:INTERMED DISCOVERY
Liver x receptor agonists and uses thereof
InactiveUS20160324981A1Modulate its functionImmunoglobulins against cell receptors/antigens/surface-determinantsPharmaceutical non-active ingredientsTreatment effectAntibody fragments
Disclosed herein are antibody drug conjugates having an antibody or antibody fragment that binds a cell surface molecule on a target cell, wherein the target cell is a lymphocyte and a therapeutic agent that has a therapeutic effect in a subject in need thereof. Further disclosed herein are antibody drug conjugates having an antibody or antibody fragment that binds a cell surface molecule on a target cell; and a therapeutic agent that binds an intracellular moiety of the target cell. These antibody drug conjugates may be used for treating cardiovascular diseases.
Owner:THE SCRIPPS RES INST
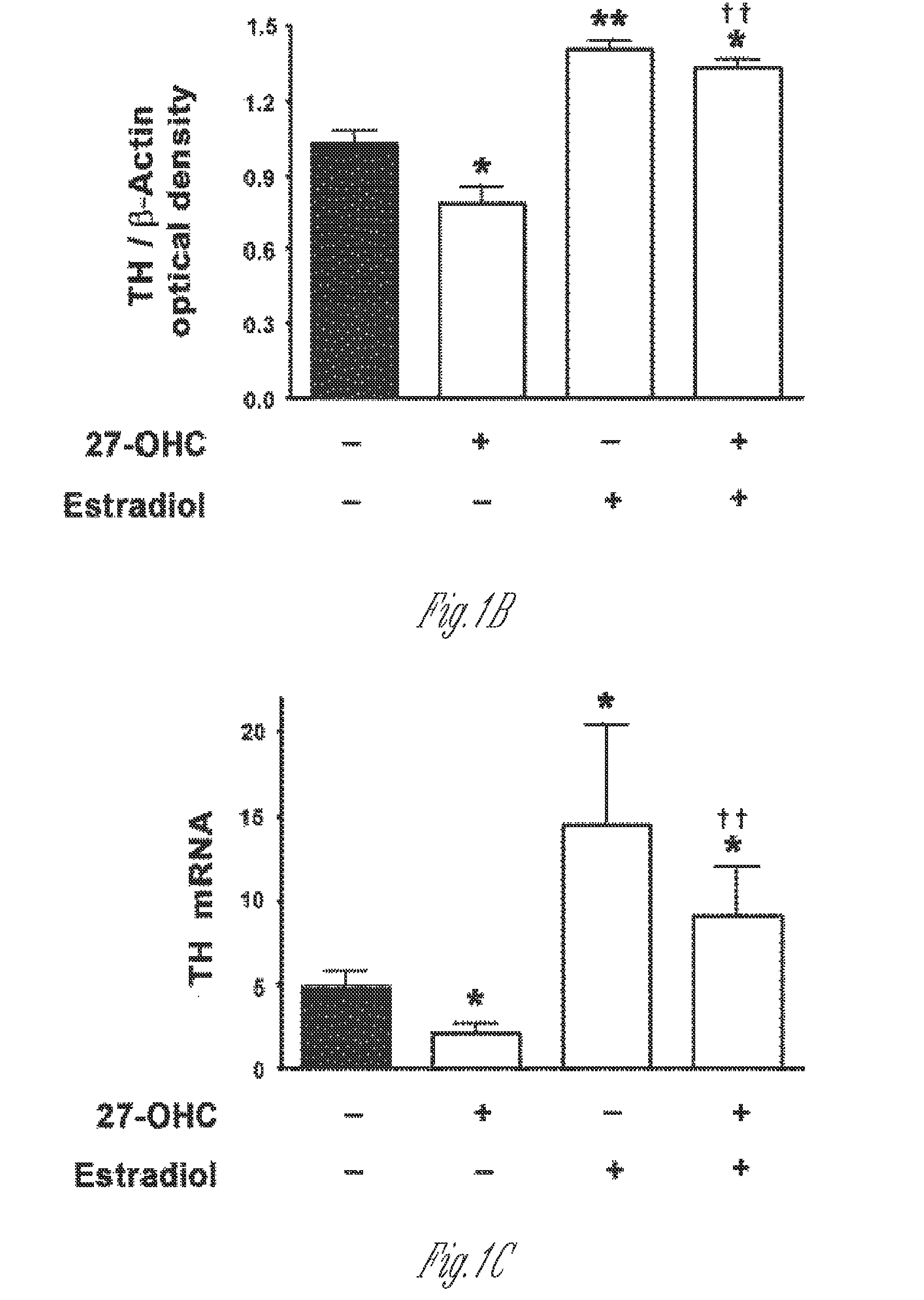
Combination of liver x receptor modulator and estrogen receptor modulator for the treatment of age-related diseases
The disclosure provides a method of treating a mammal afflicted with an age-related disorder, comprising administering to the mammal a combination of liver X receptor (LXR) modulator and estrogen receptor (ER) modulator, in an amount effective to treat the mammal. Further disclosed are the LXR modulators and ER modulators used in the combination therapy.
Owner:UNIVERSITY OF NORTH DAKOTA
Benzimidazole compounds
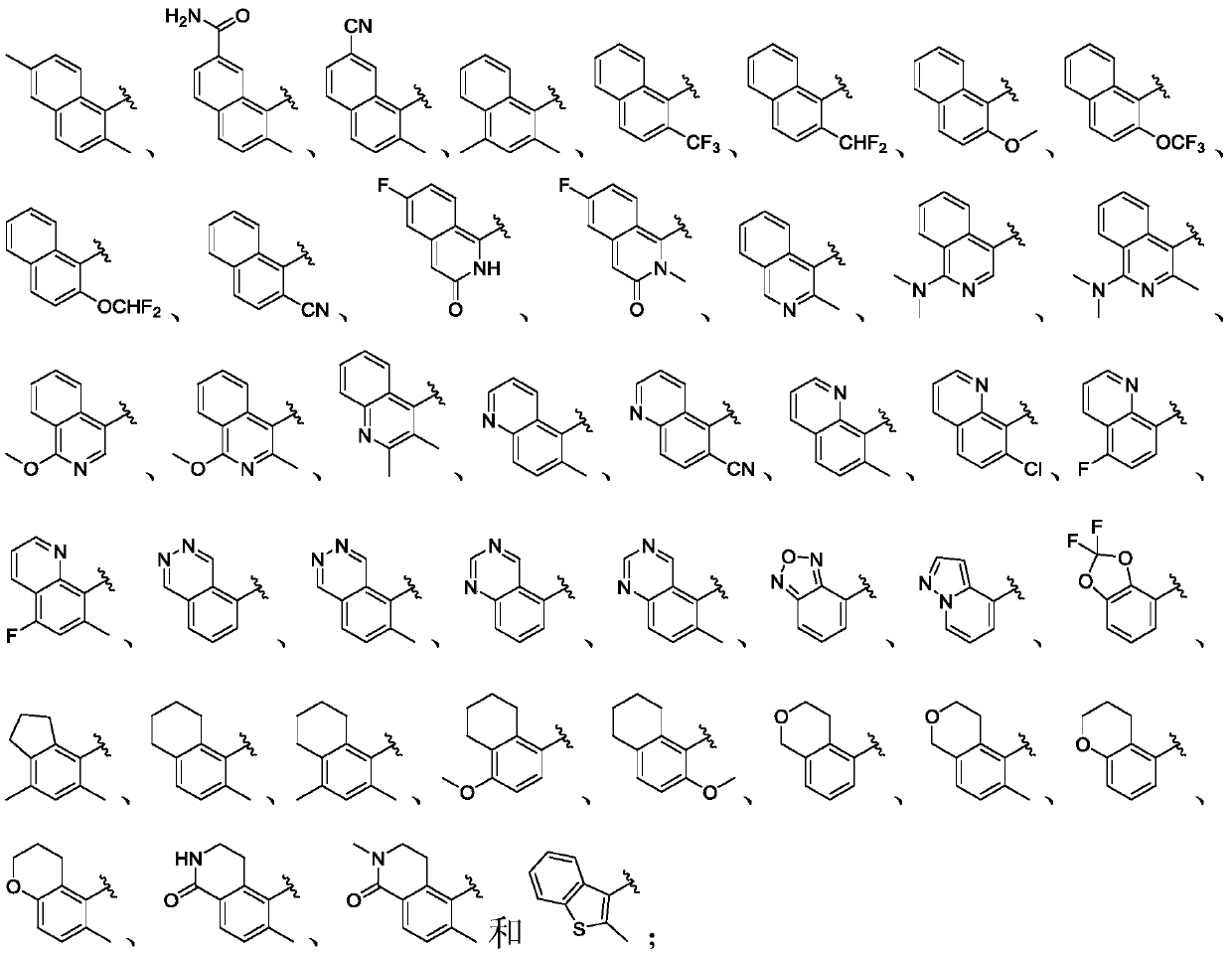
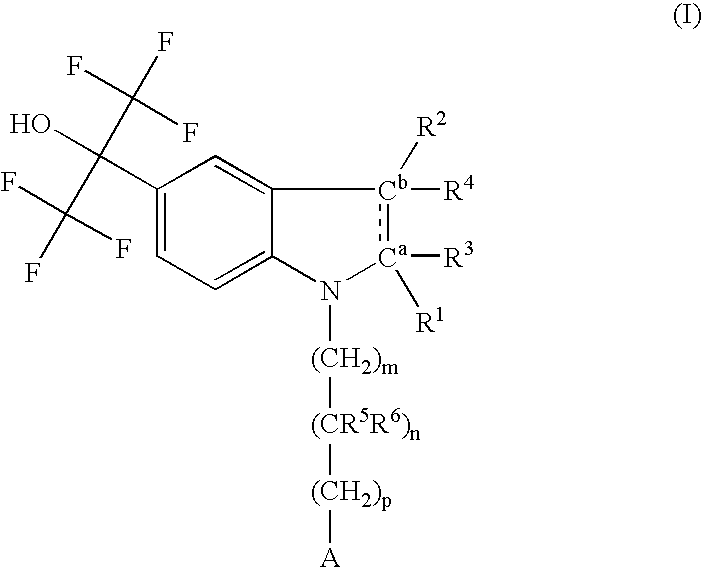
This invention relates generally to benzimidazole-based modulators of Liver X receptors (LXRs) and related methods (Formula I). wherein R2 is C6-C10 aryl or heteroaryl including 5-10 atoms, each of which is: (i) substituted with 1 R7, and (ii) optionally substituted with from 1-5 Re; and R1, R3, R4, R5, R6, R7, and Re are defined herein.
Owner:WYETH LLC
Purine derivatives as liver X receptor agonists
Owner:GLAXO GRP LTD
Pyrrole-2,5-dione derivatives as Liver X receptor modulators
The present invention relates to certain novel compounds of the Formula Ito processes for preparing such compounds, to their the utility in modulation of nuclear hormone receptors Liver X Receptor (LXR)α(NR1H3) and / or β(NR1H2) and in treating and / or preventing clinical conditions including cardiovascular diseases such as atherosclerosis; inflammatory diseases, Alzheimer's disease, lipid disorders (dyslipidemias) whether or not associated with insulin resistance, type 2 diabetes and other manifestations of the metabolic syndrome, to methods for their therapeutic use and to pharmaceutical compositions containing them.
Owner:ASTRAZENECA AB
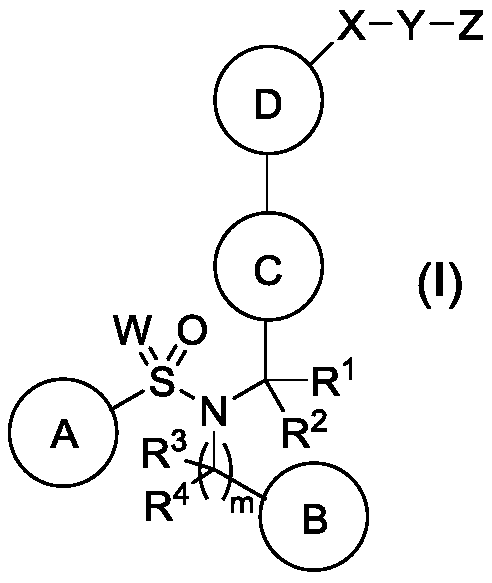
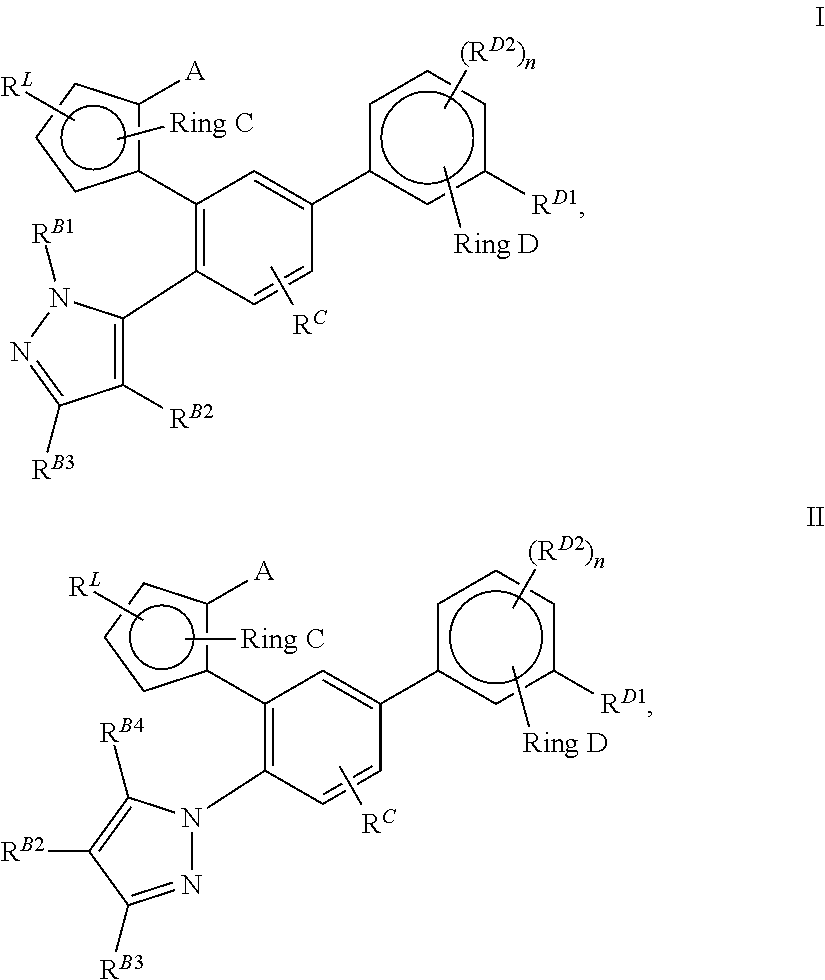
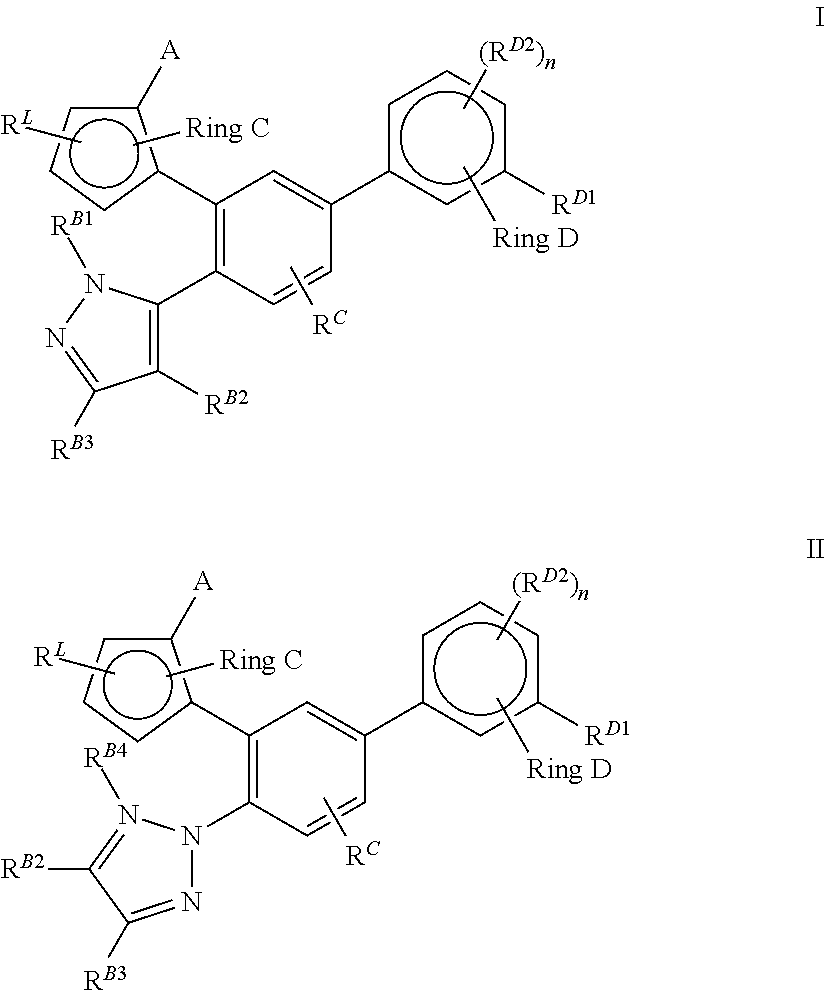
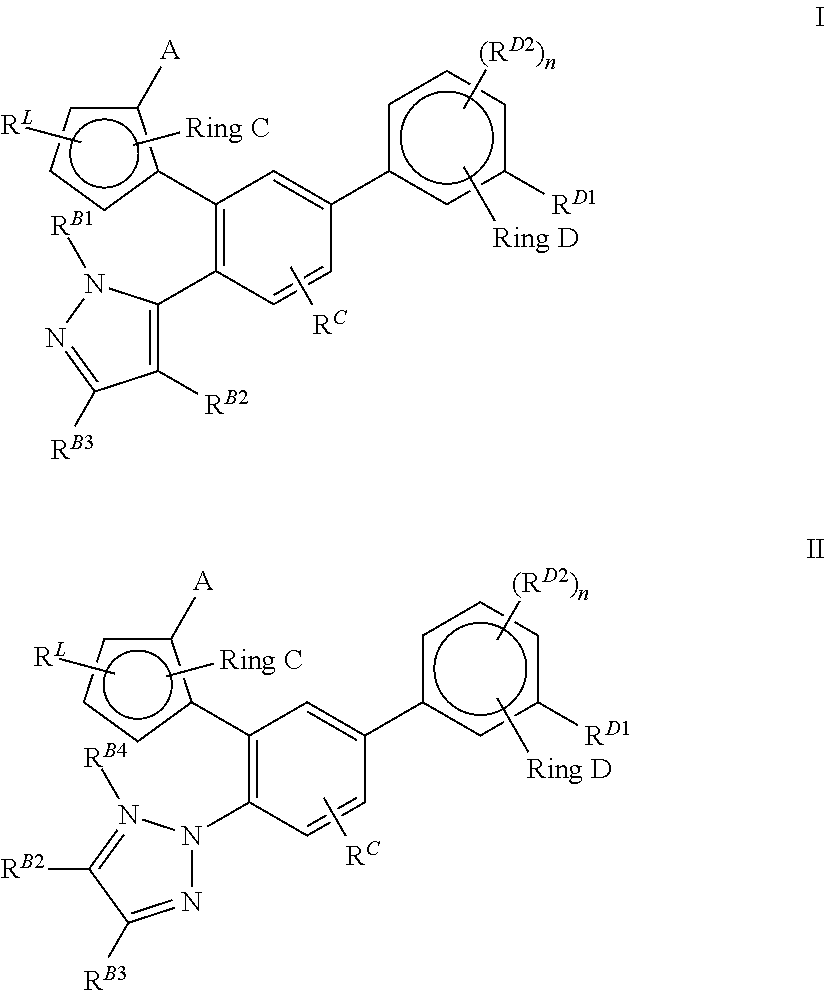
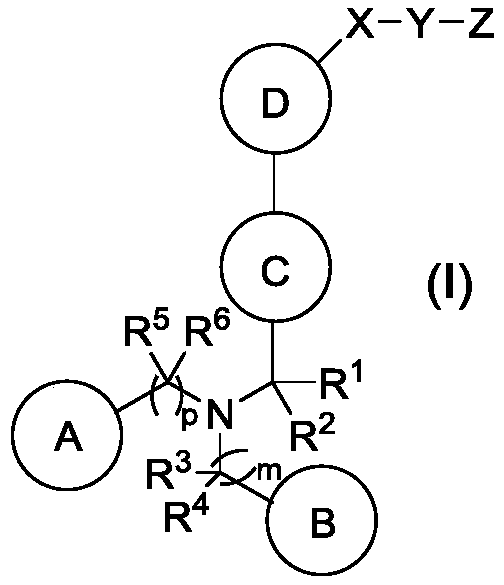
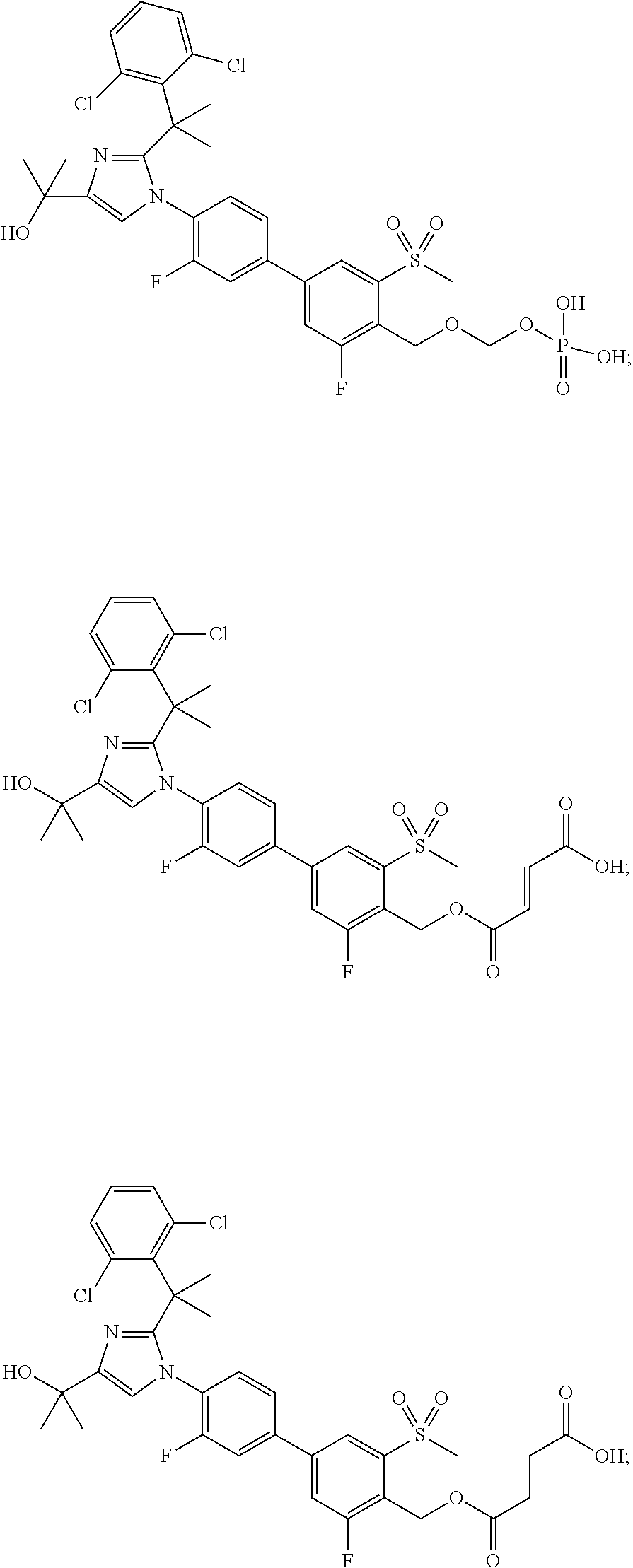
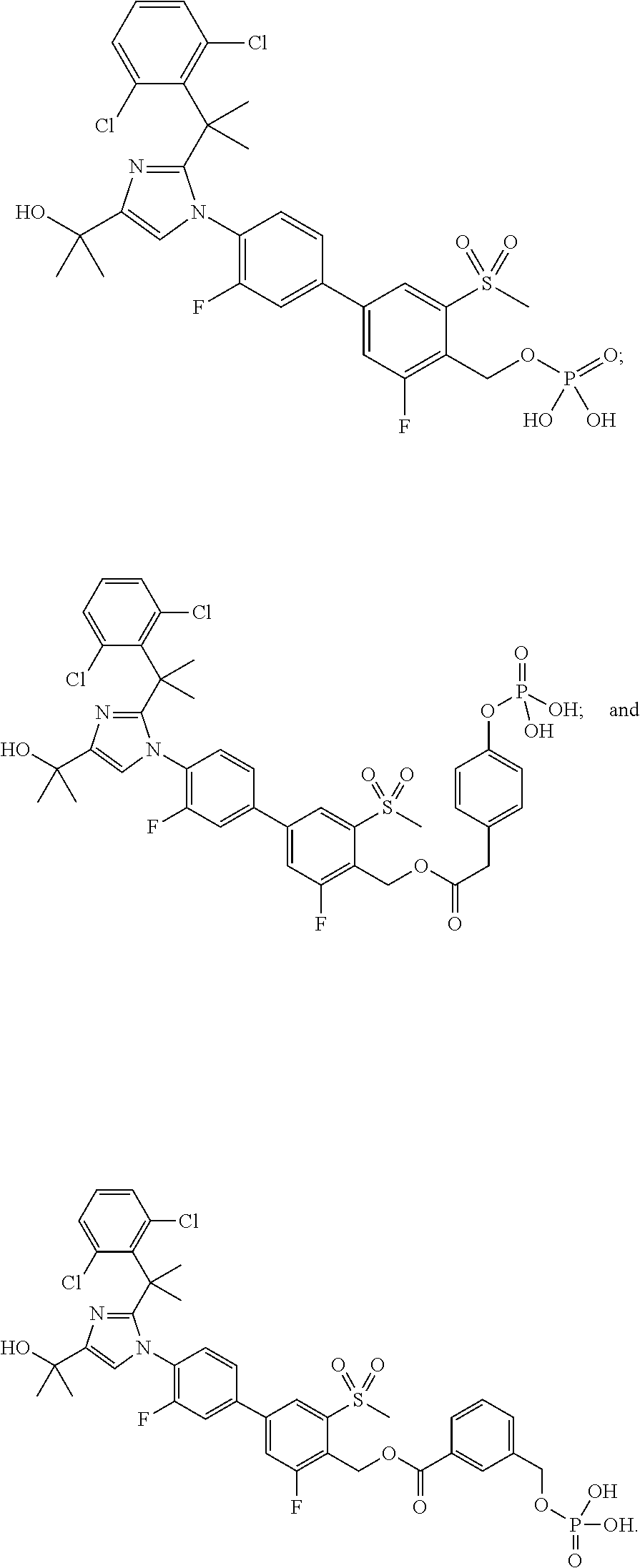
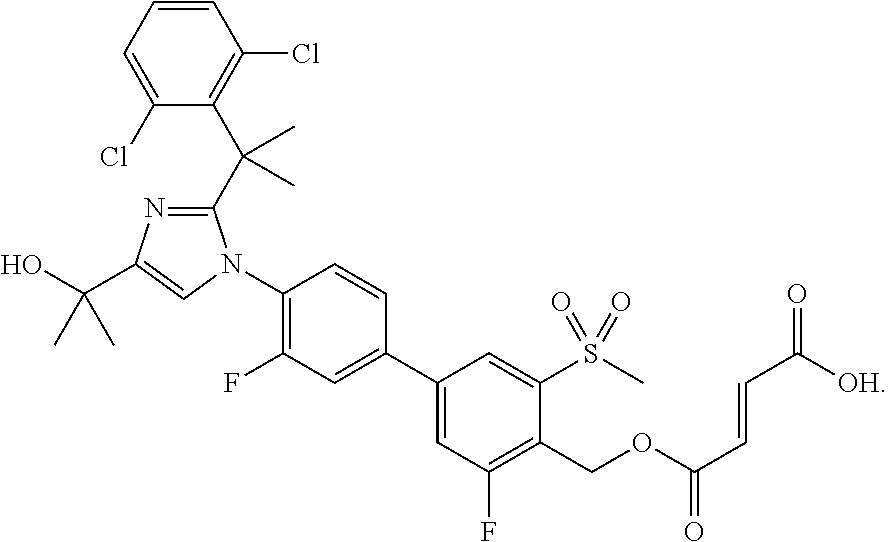
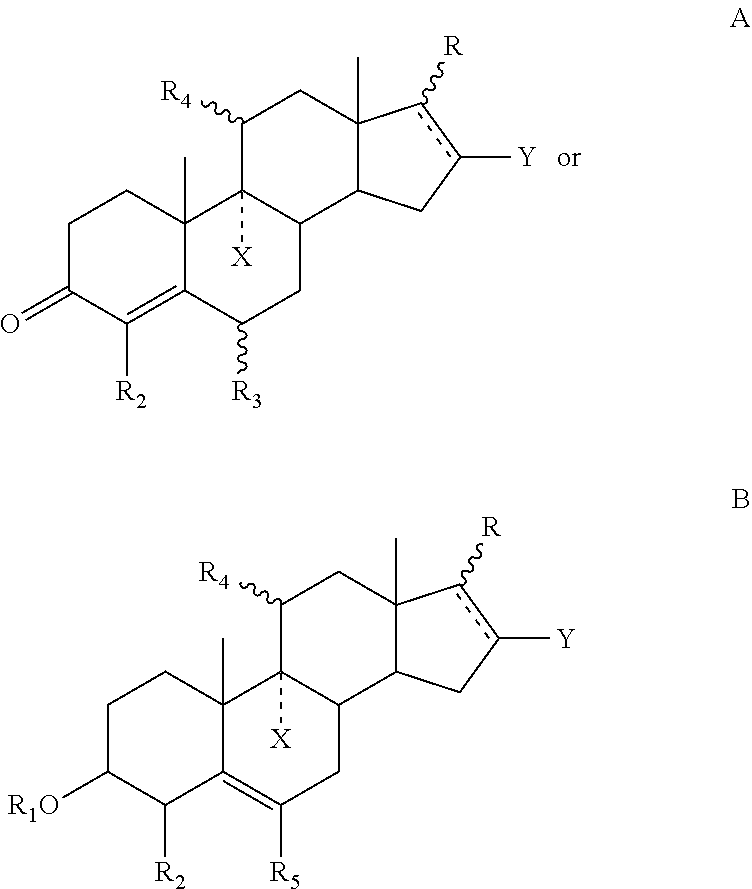
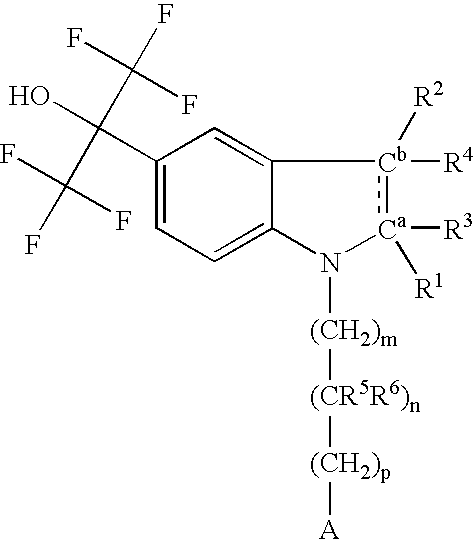
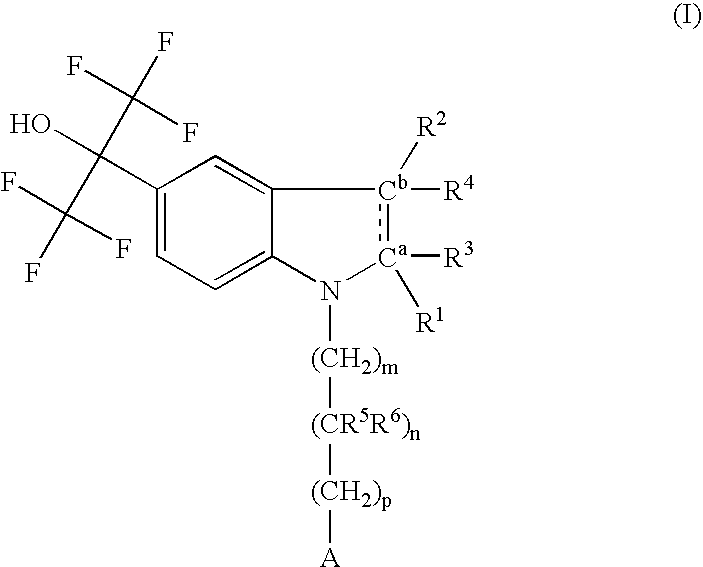
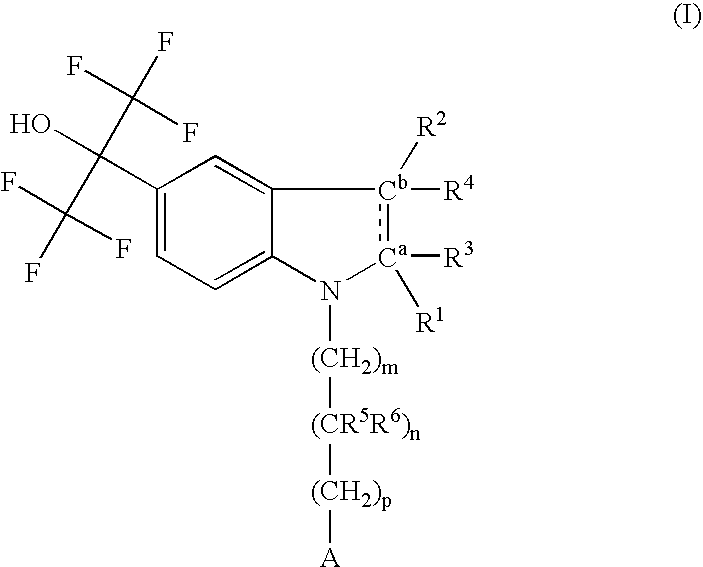
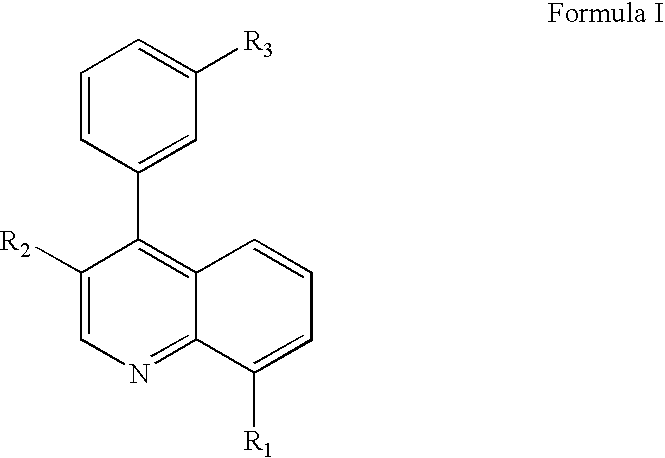
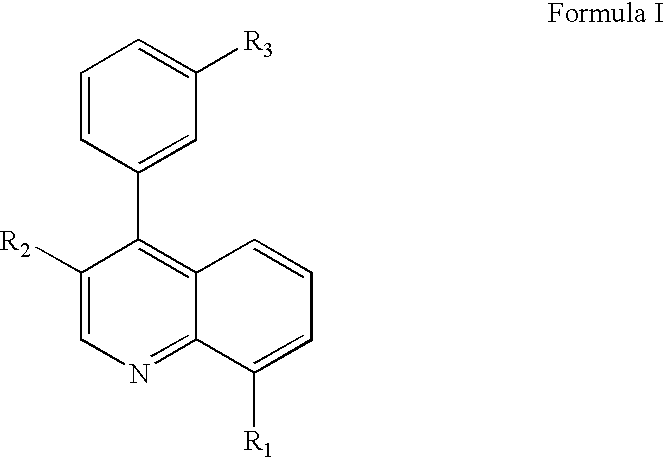
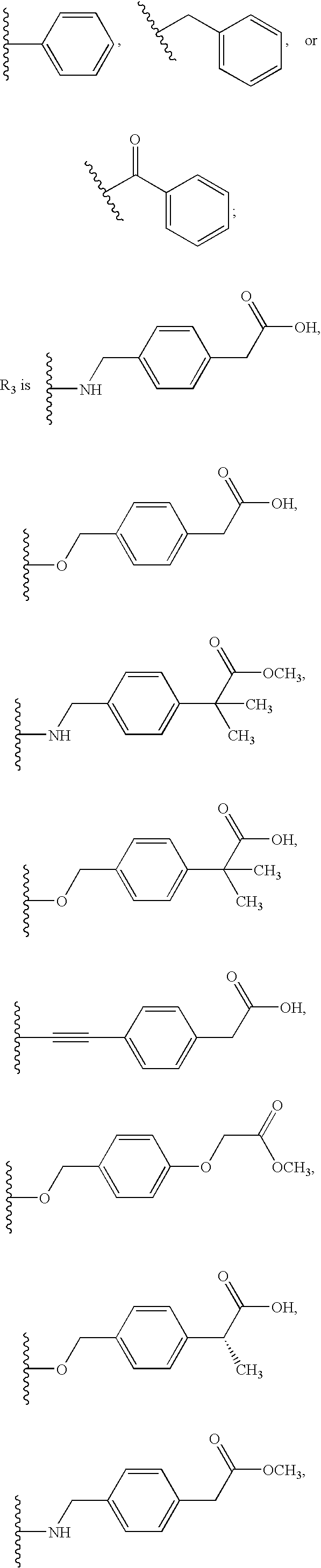
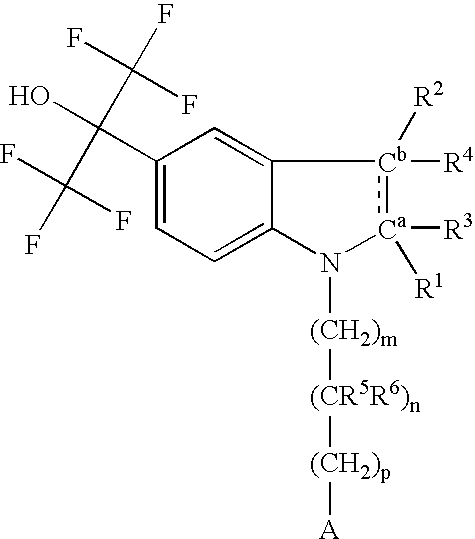
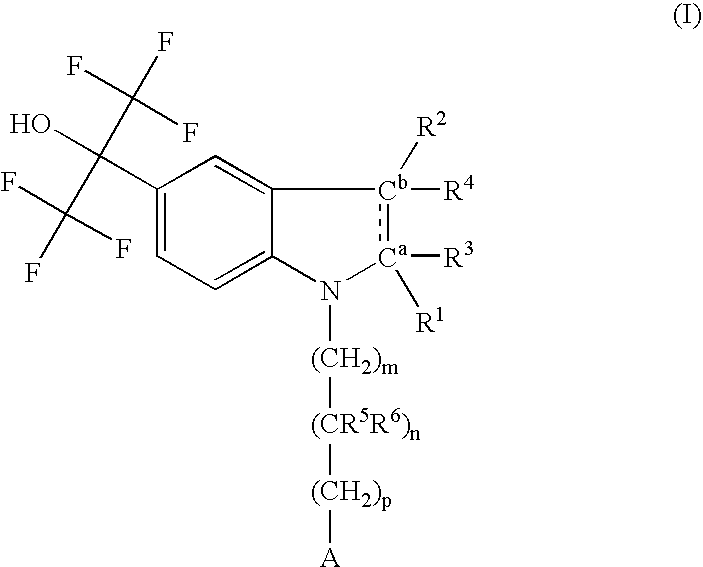
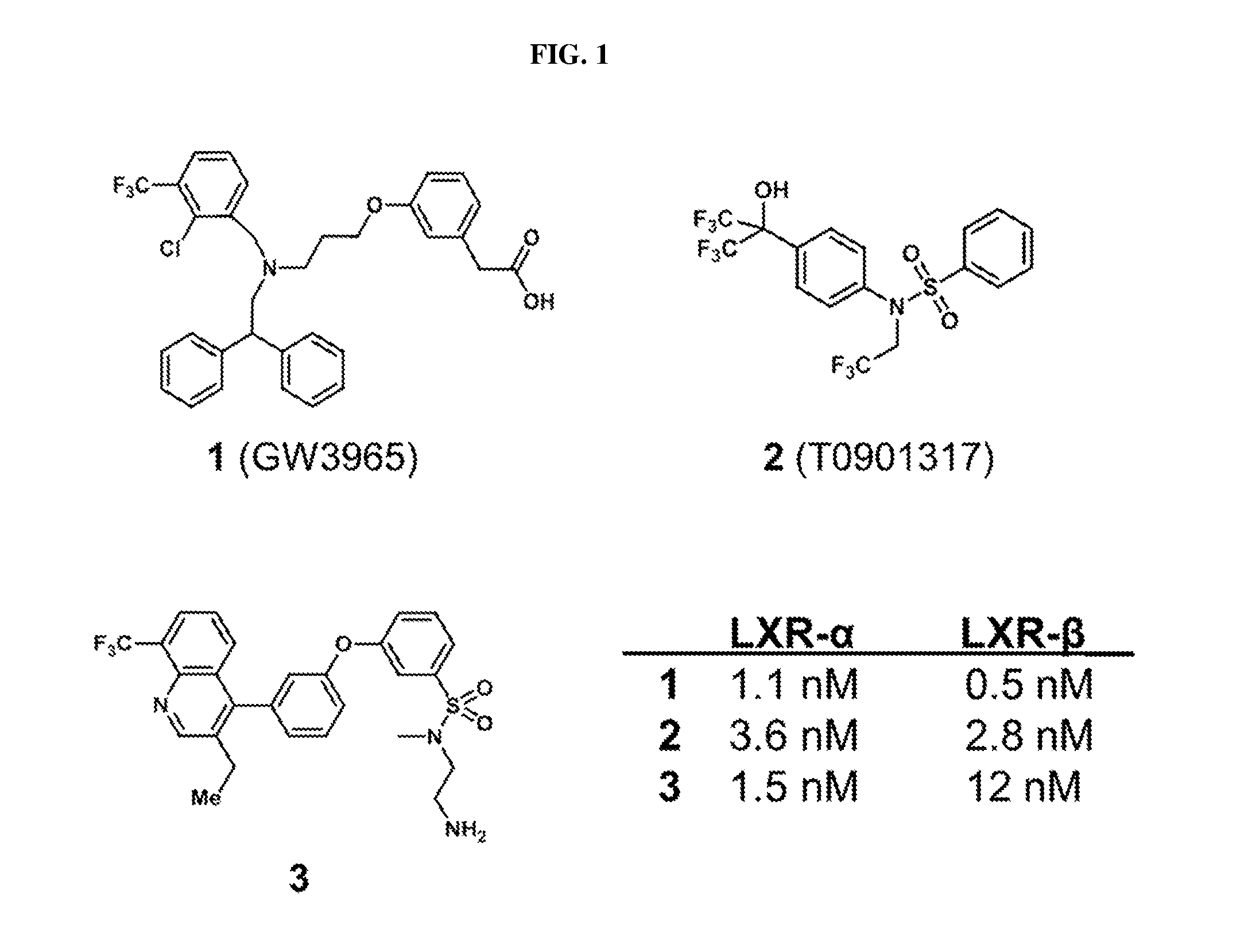
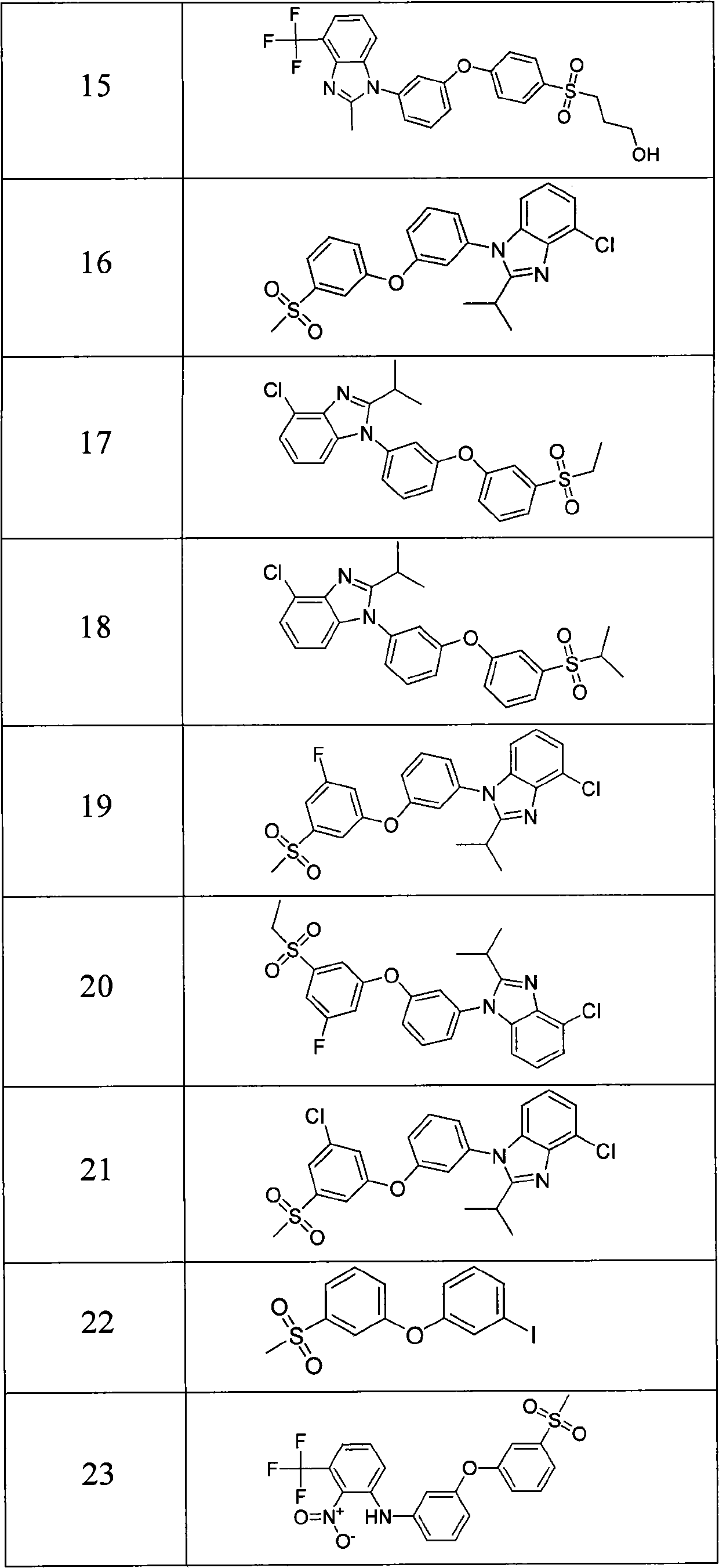
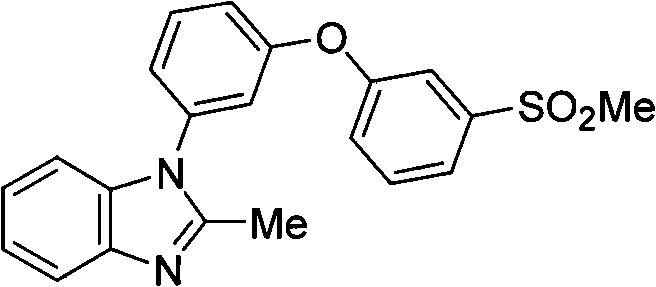
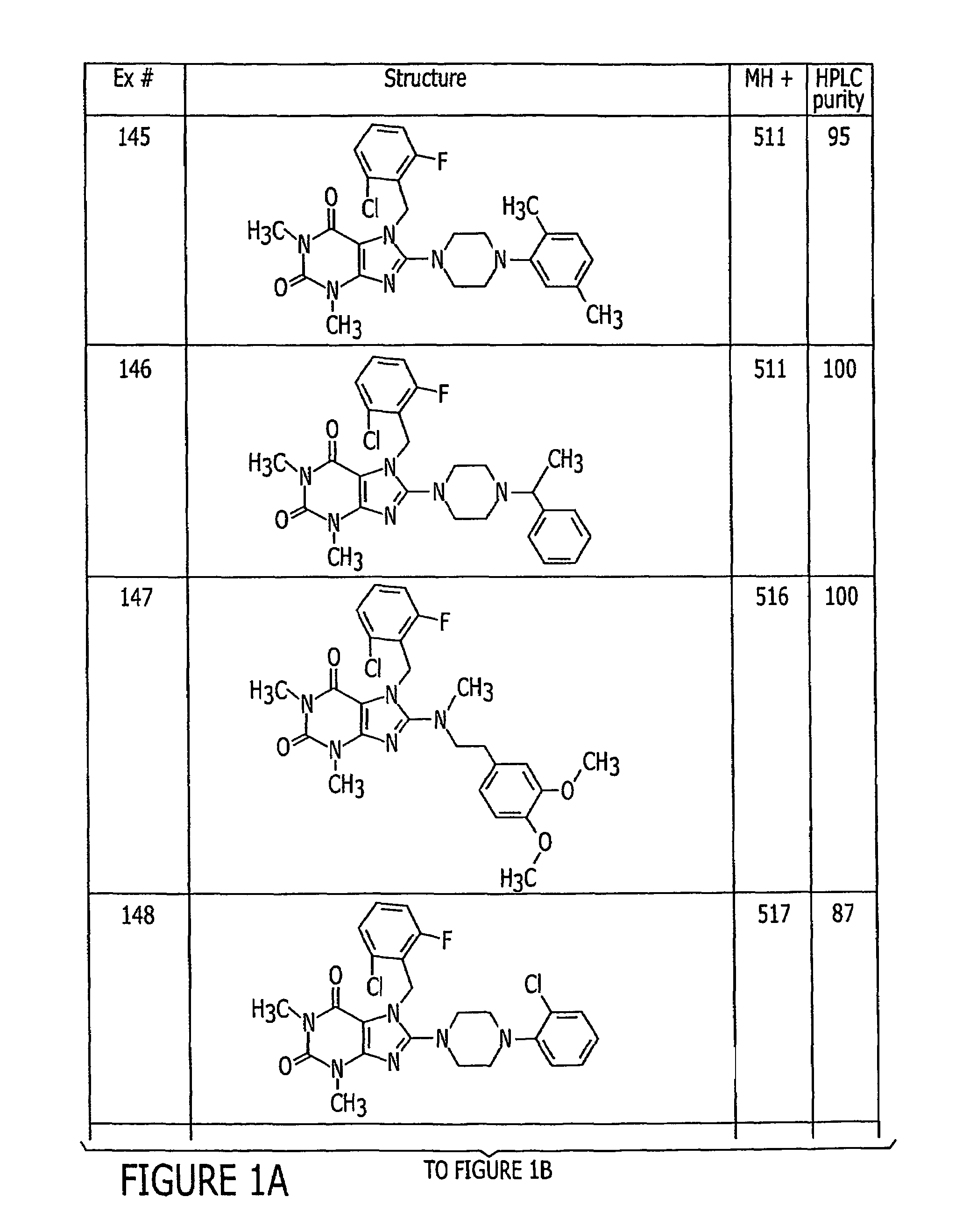
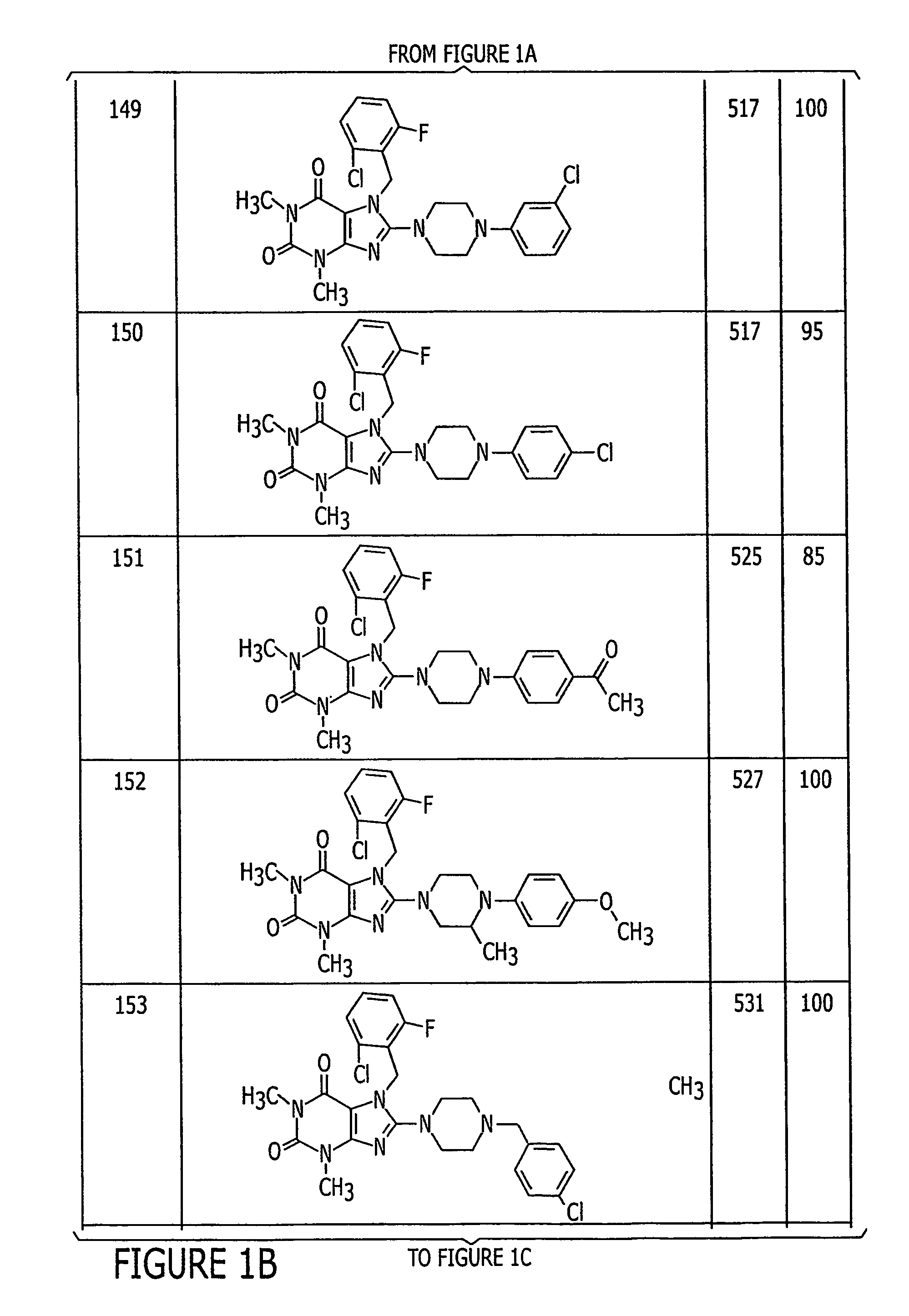
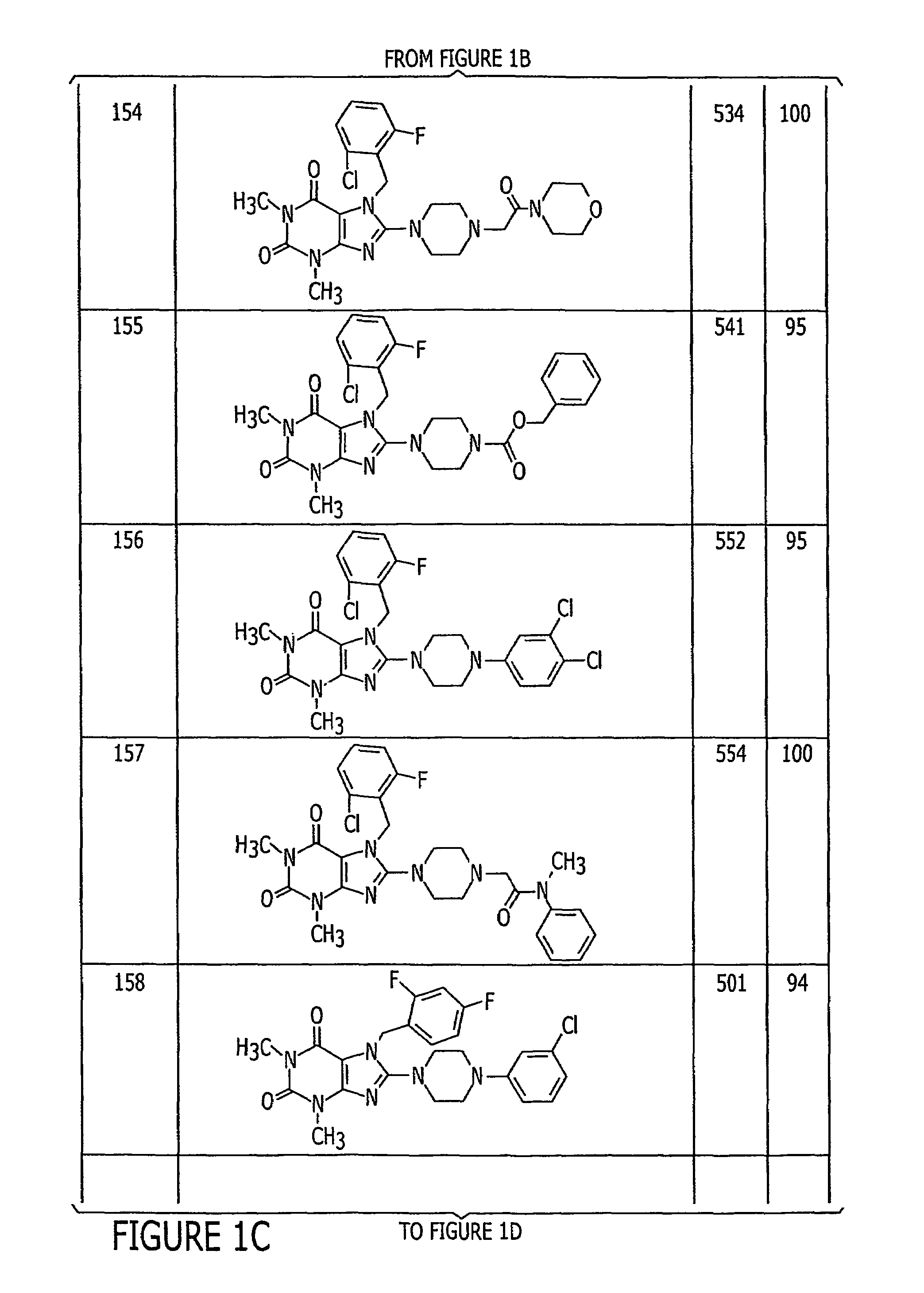
Pyrazolo [1,5-A] pyrimidine compounds
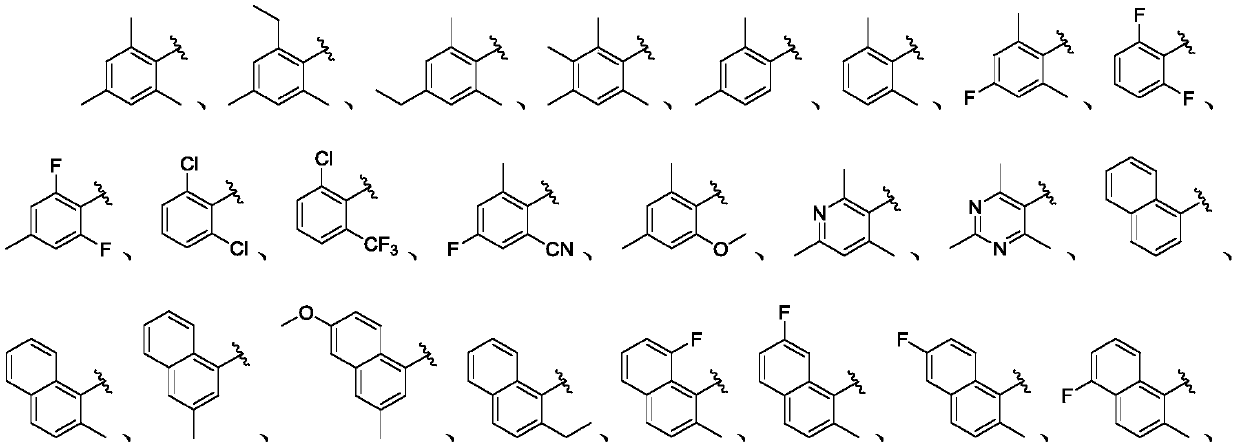
This invention relates generally to pyrazolo [1,5-a] pyrimidine-based modulators of Liver X receptors (LXRs) having formula (I) and related methods: Formula (I)wherein R2 is C6-C10 aryl or heteroaryl including 5-10 atoms, each of which is: (i) substituted with 1 R6, and (ii) optionally substituted with from 1-5 Re; and R1, R3, R4, R5, R6 and Re are defined herein.
Owner:WYETH LLC
Novel use of liver X receptor agonists
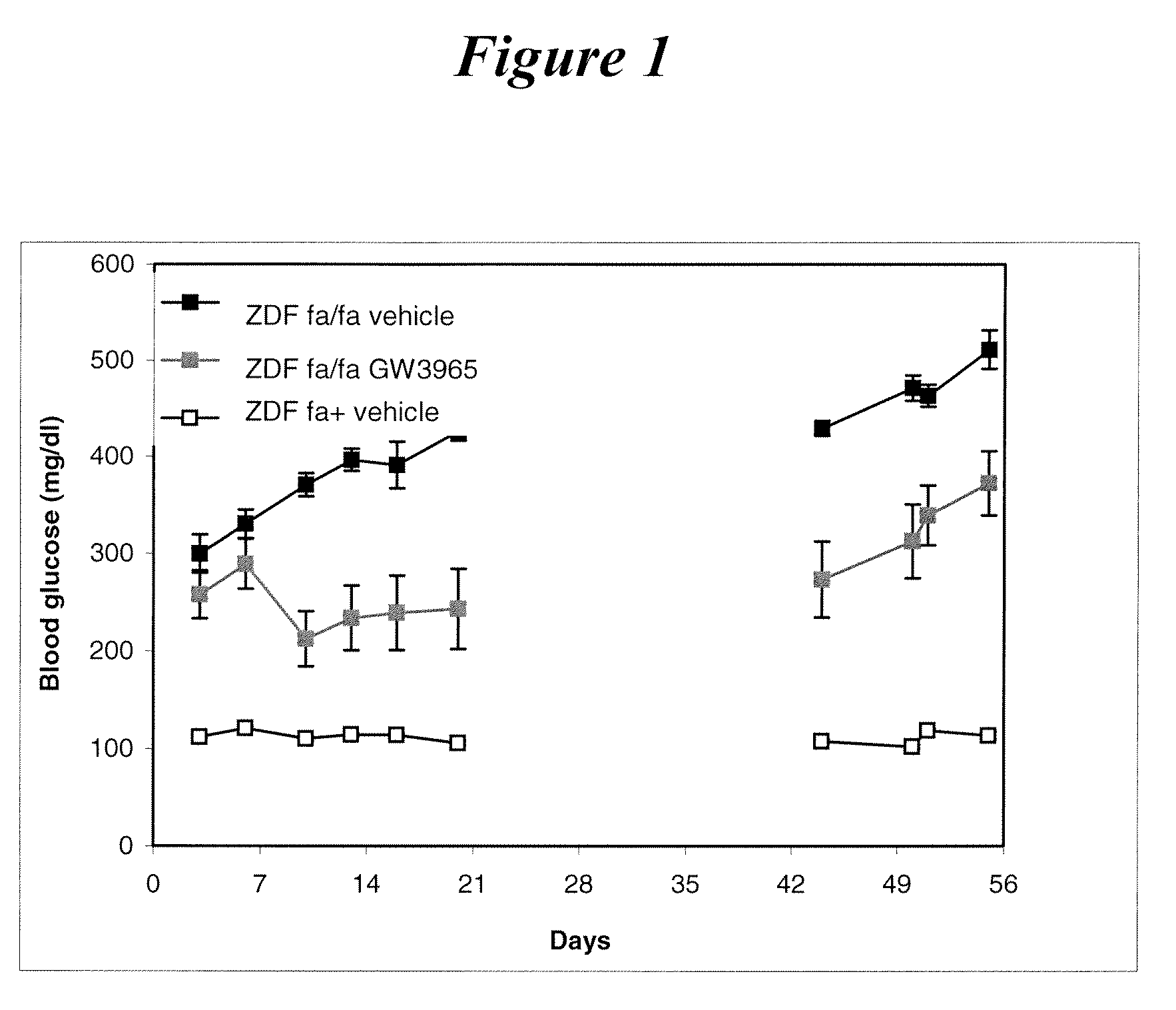
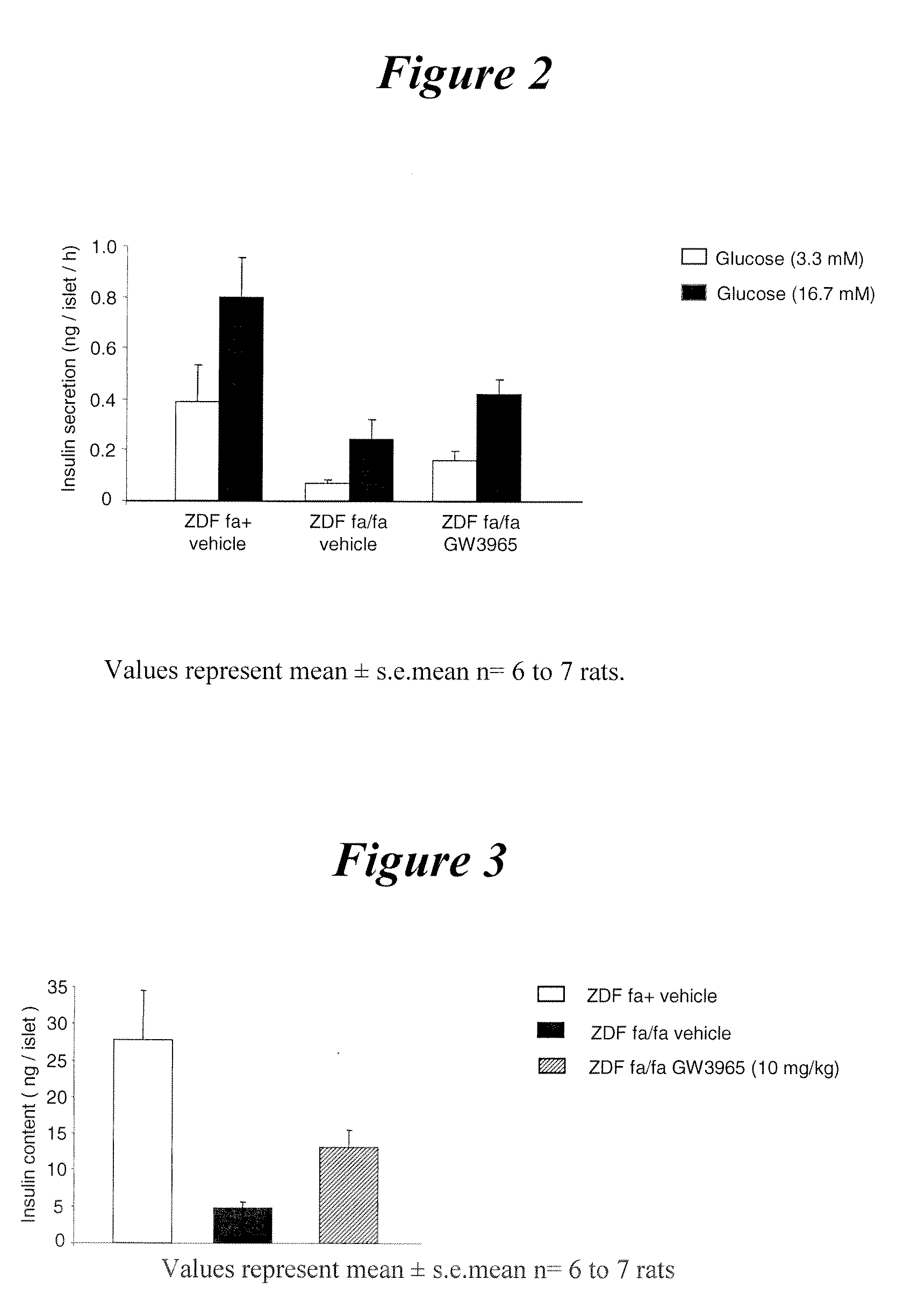
InactiveUS20090118306A1Improvement of diabetic stateReduce glycemiaBiocideHalogenated hydrocarbon active ingredientsLiver X receptorDrug
The present invention generally relates to a novel therapeutical use of liver X receptor (LXR) agonsits. More specifically, the present invention relates to the use of LXR agonist for the preparation of a medicament useful for the treatment and / or the prevention of a disease associated with beta cells degeneration, such as diabetes, and a method for increasing ex vivo viability of pancreatic islet cells, comprising contacting said islet cells with a LXR agonist.
Owner:LABES FOURNIER
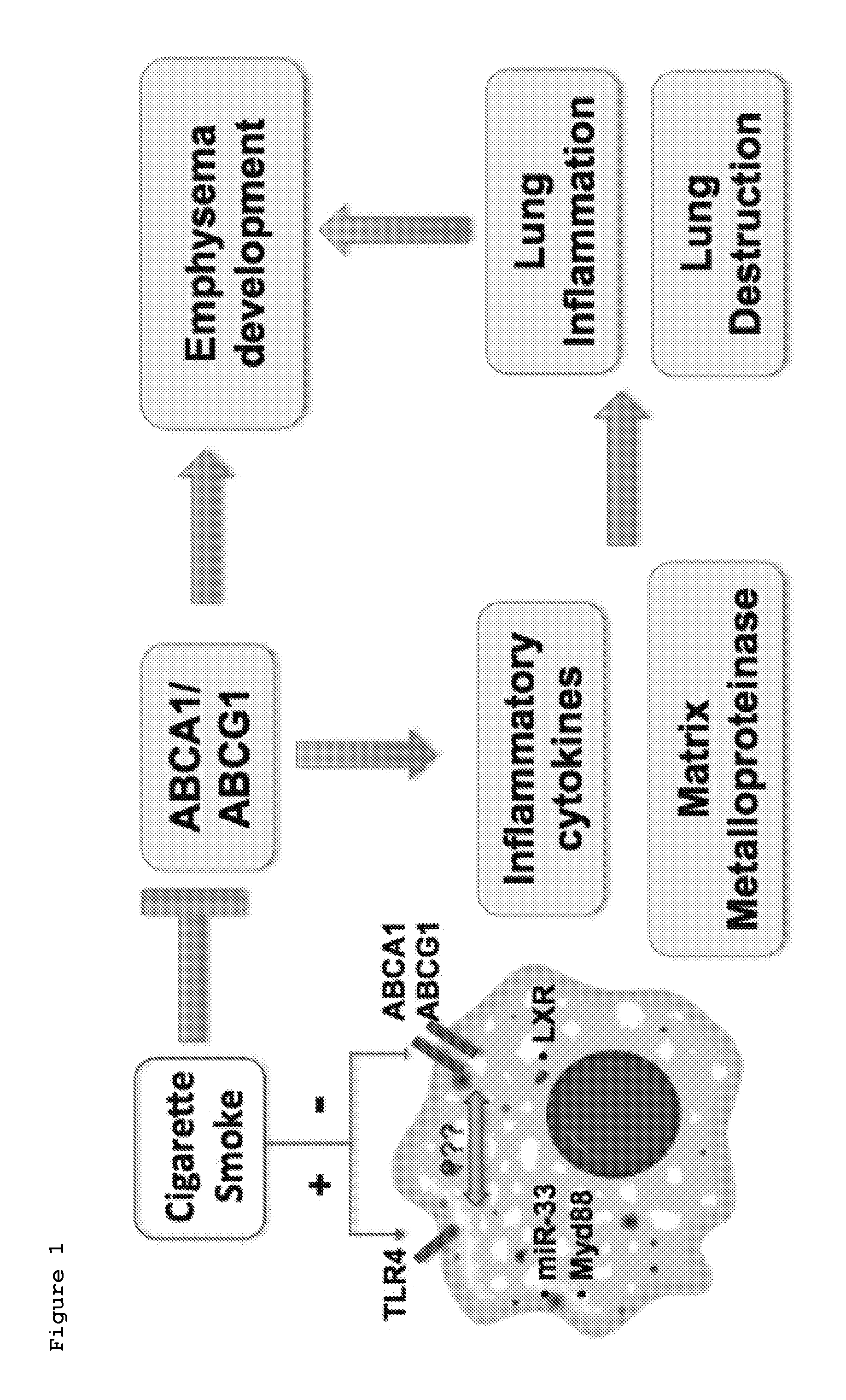
Liver x receptor agonists in the treatment of emphysema
InactiveUS20160310454A1Peptide/protein ingredientsDisease diagnosisObstructive Pulmonary DiseasesAgonist
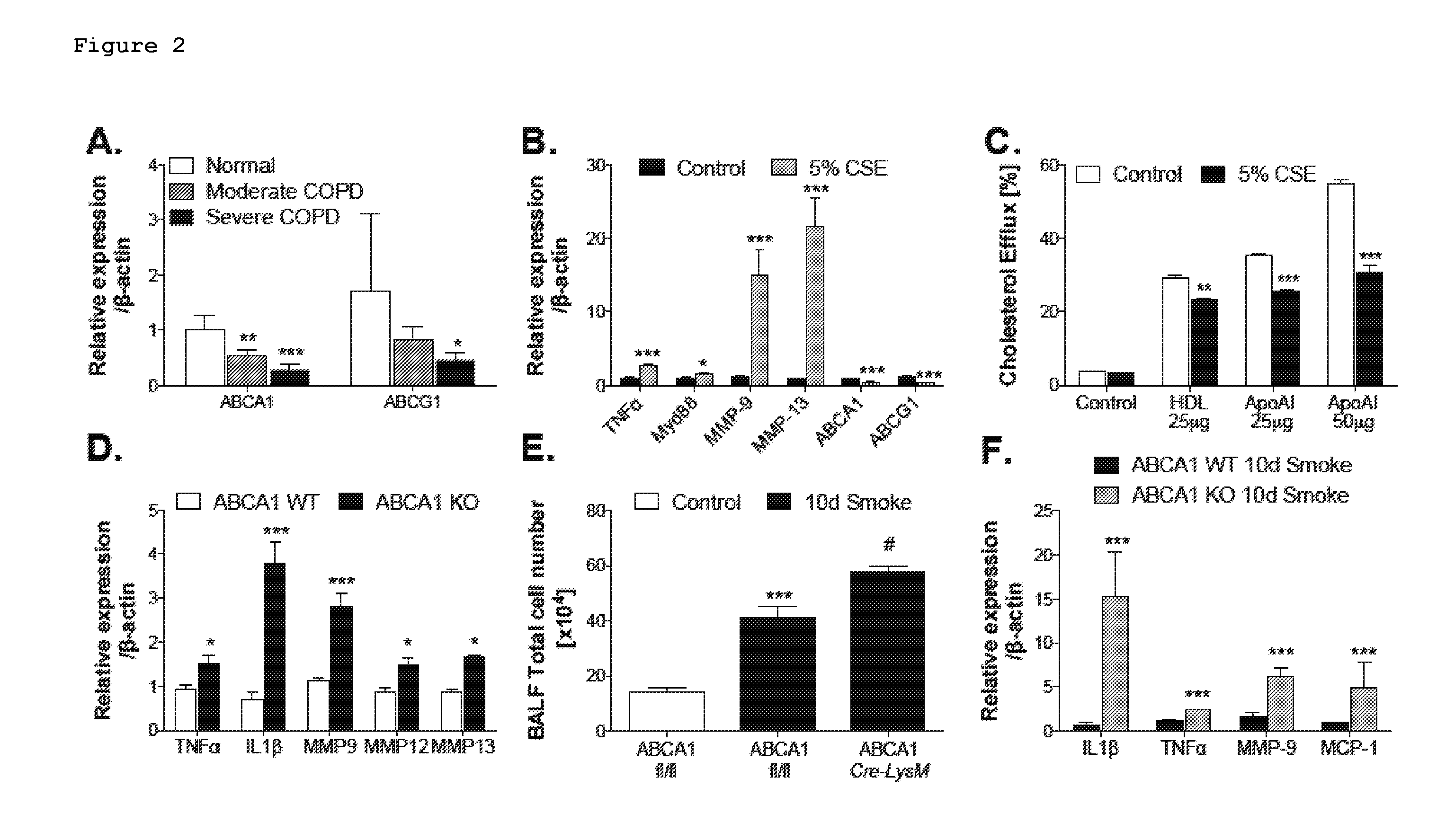
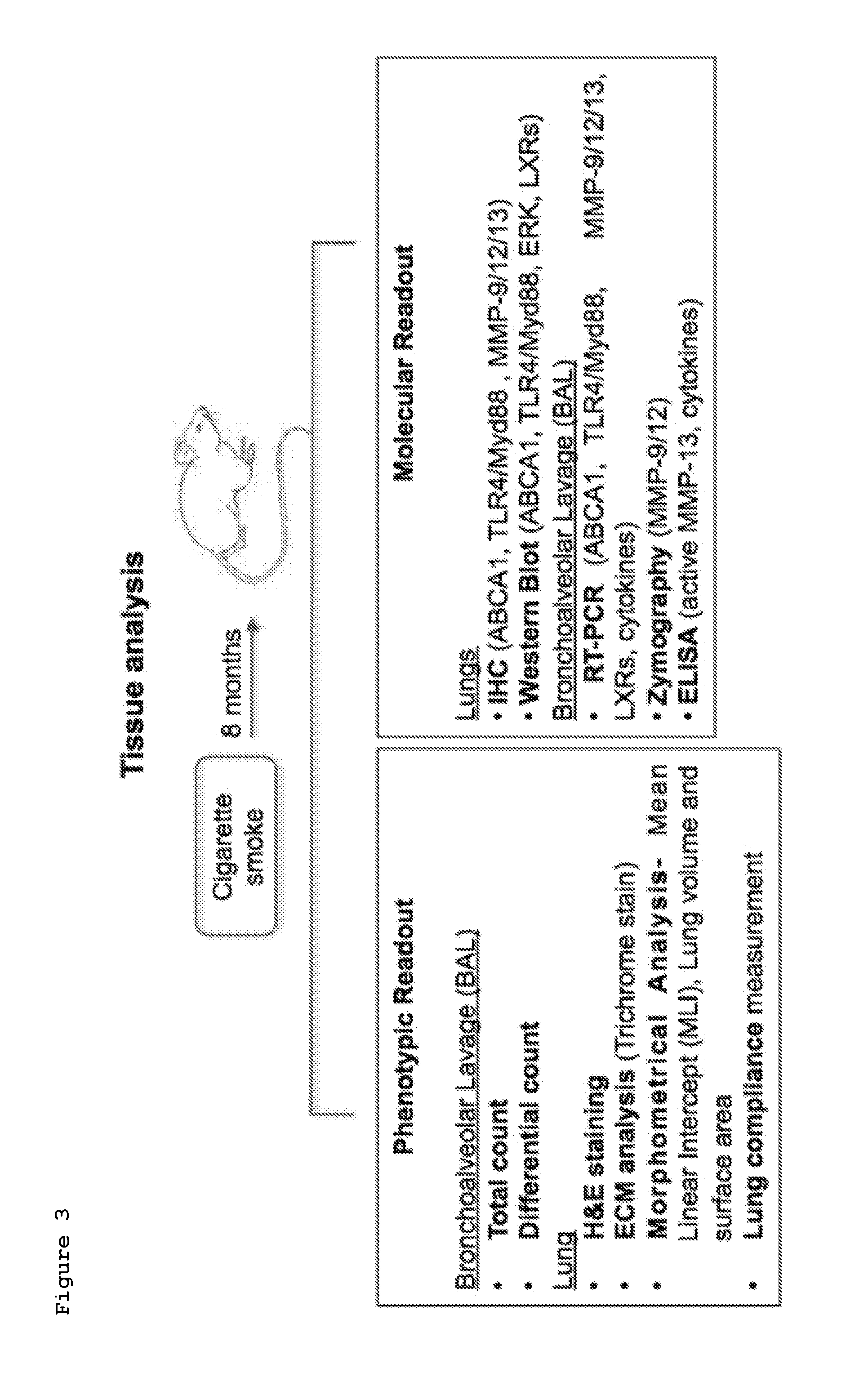
The present invention provides methods and compositions for treating a subject afflicted with chronic obstructive pulmonary disease (COPD) which comprise a i) a Liver X receptor (LXR) agonist, ii) a miR-33 antagonist, or iii) a TLR4 / Myd88 pathway antagonist. The present invention also provides methods and compositions for use in prophylactically treating a subject for chronic obstructive pulmonary disease (COPD) which comprise i) a Liver X receptor (LXR) agonist, ii) a miR-33 antagonist, or iii) a TLR4 / Myd88 pathway antagonist.
Owner:DARMIENTO JEANINE +2
Lipid-lowering drug comprising wild chrysanthemum extract and preparation method of wild chrysanthemum extract
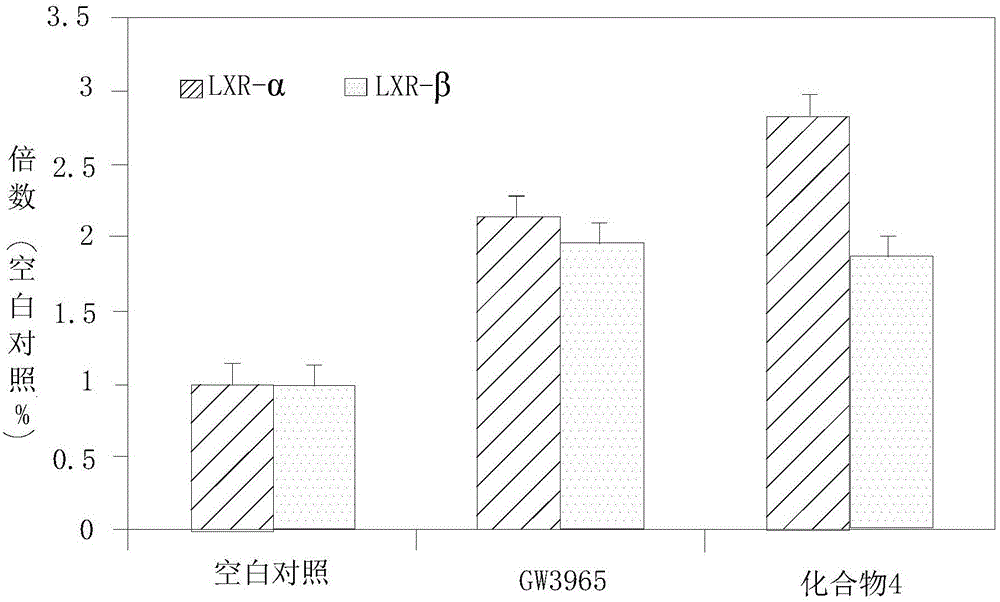
InactiveCN106344558AExcellent stimulant effectStrong stimulant effectOrganic active ingredientsMetabolism disorderPositive controlSrebp 1c
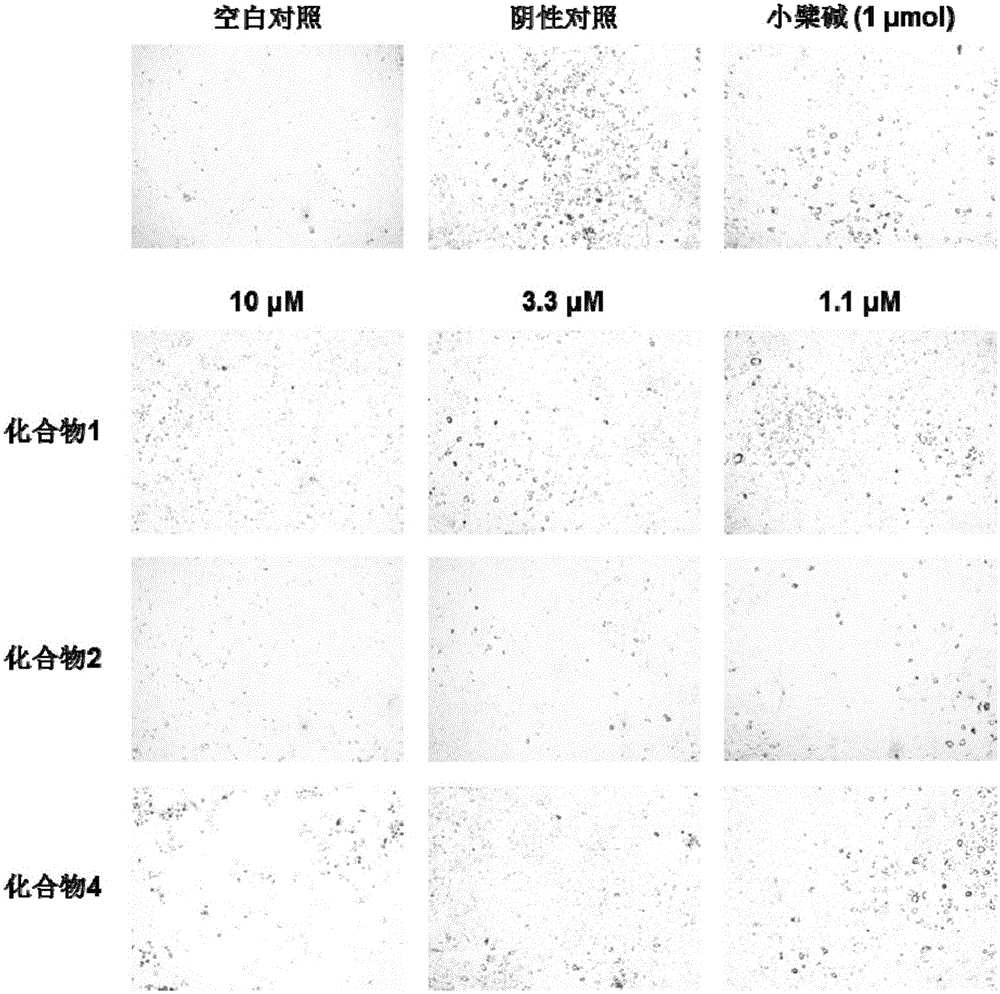
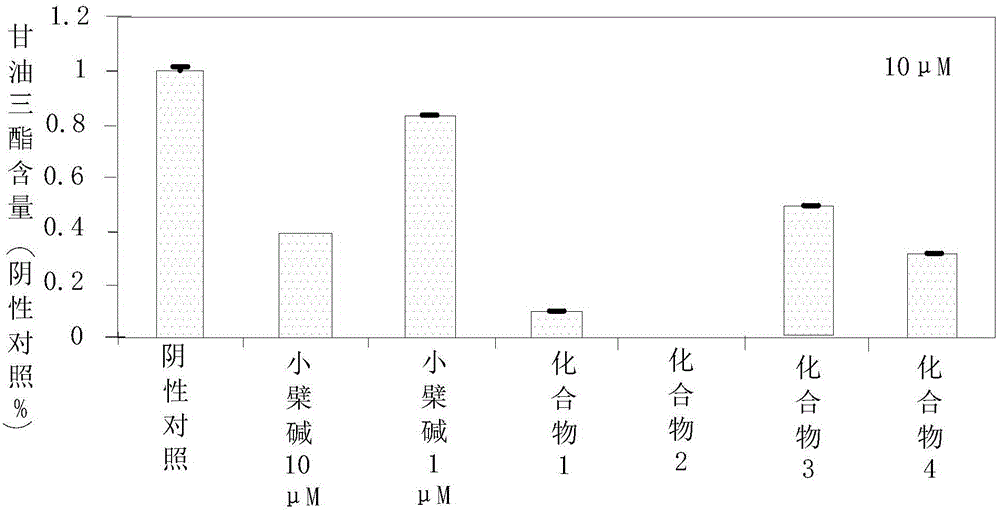
The invention relates to the technical field of lipid-lowering drugs, and specifically relates to a lipid-lowering drug comprising a wild chrysanthemum extract and a preparation method of the wild chrysanthemum extract. The wild chrysanthemum extract in the lipid-lowering drug comprises a sesquiterpenoids compound disclosed by the invention, wherein the sesquiterpenoids compound remarkably inhibits the accumulation of triglyceride and total fat in the differentiation process of preadipocyte 3T3-L1, the inhibiting effect is better than that of berberine, a positive control compound; liver X receptors alpha and beta (LXRalpha and LXRbeta) are also remarkably excited, the exciting effect is better than that of an LXR agonist tool molecule GW3965, and particularly, the exciting effect of a compound 4 is remarkably better than that of GW3965. In addition, the sesquiterpenoids compound further regulates the mRNA expression quantity of transcription factors SREBP-1c, PPARgama and CEBPdelta which are of vital importance for cholesterol metabolism. Therefore, the disclosed lipid-lowering drug comprising the wild chrysanthemum extract has an excellent effect of lowering lipid, and has an excellent development prospect on the treatment of hyperlipidaemia.
Owner:广州市爱菩新医药科技有限公司
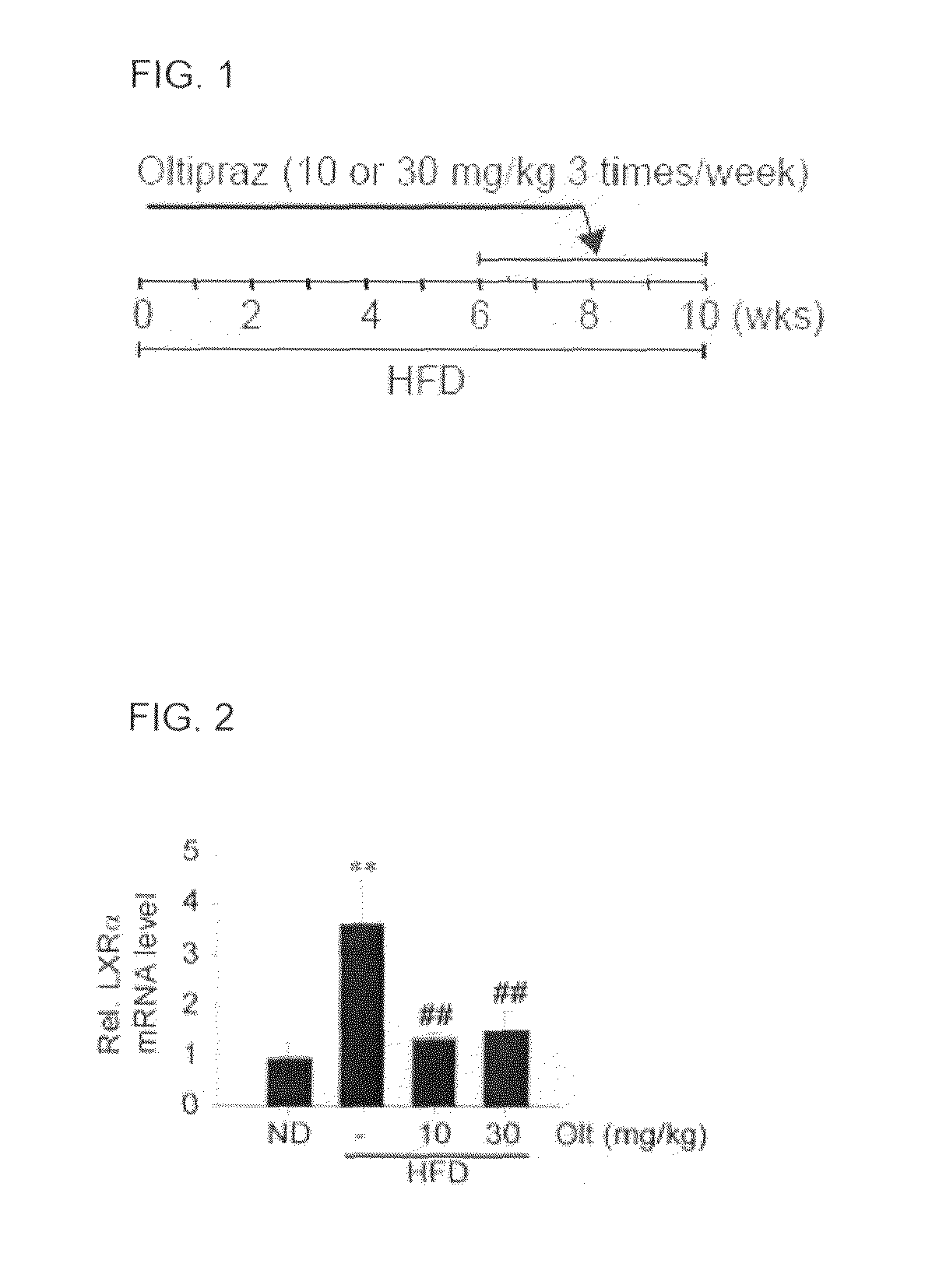
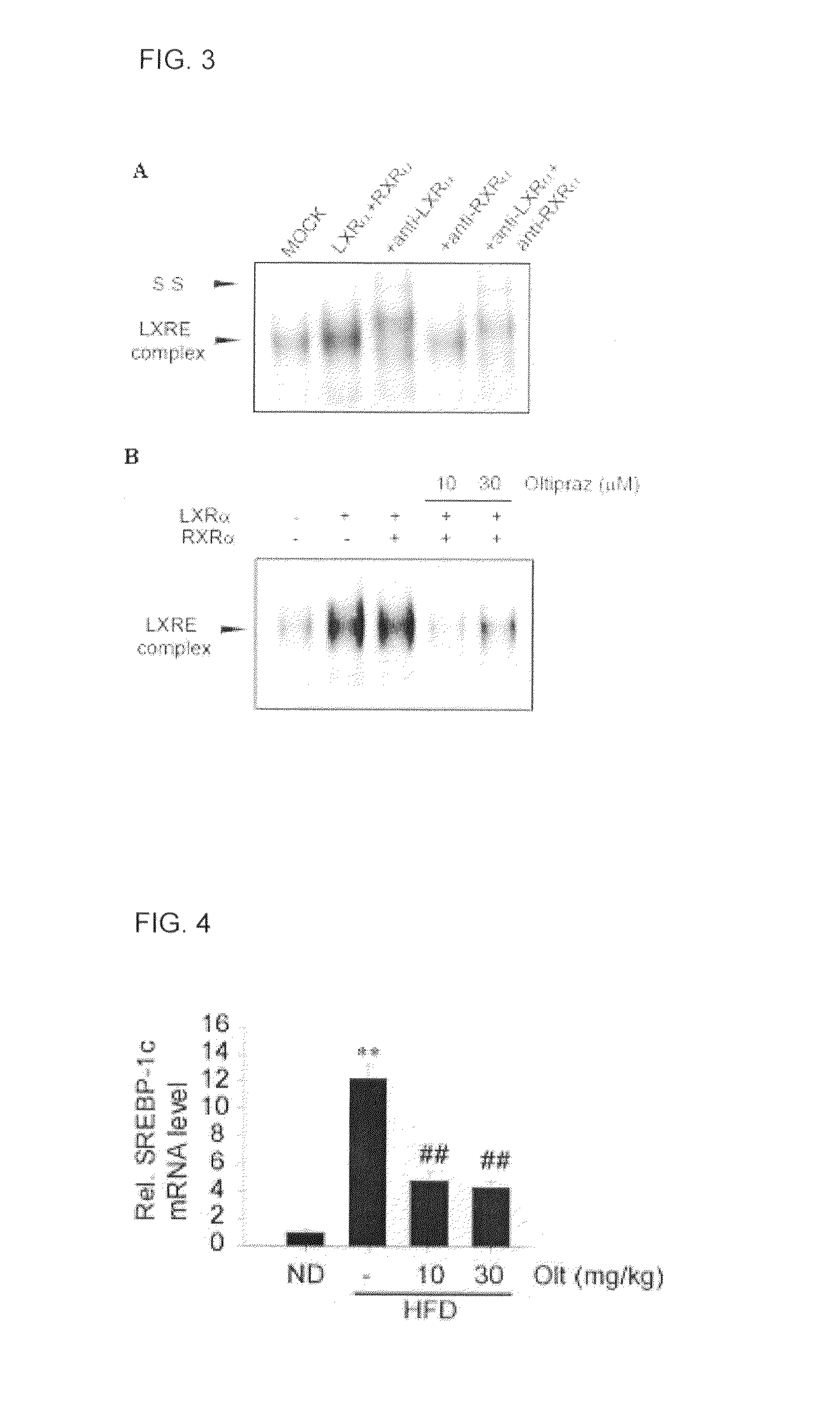
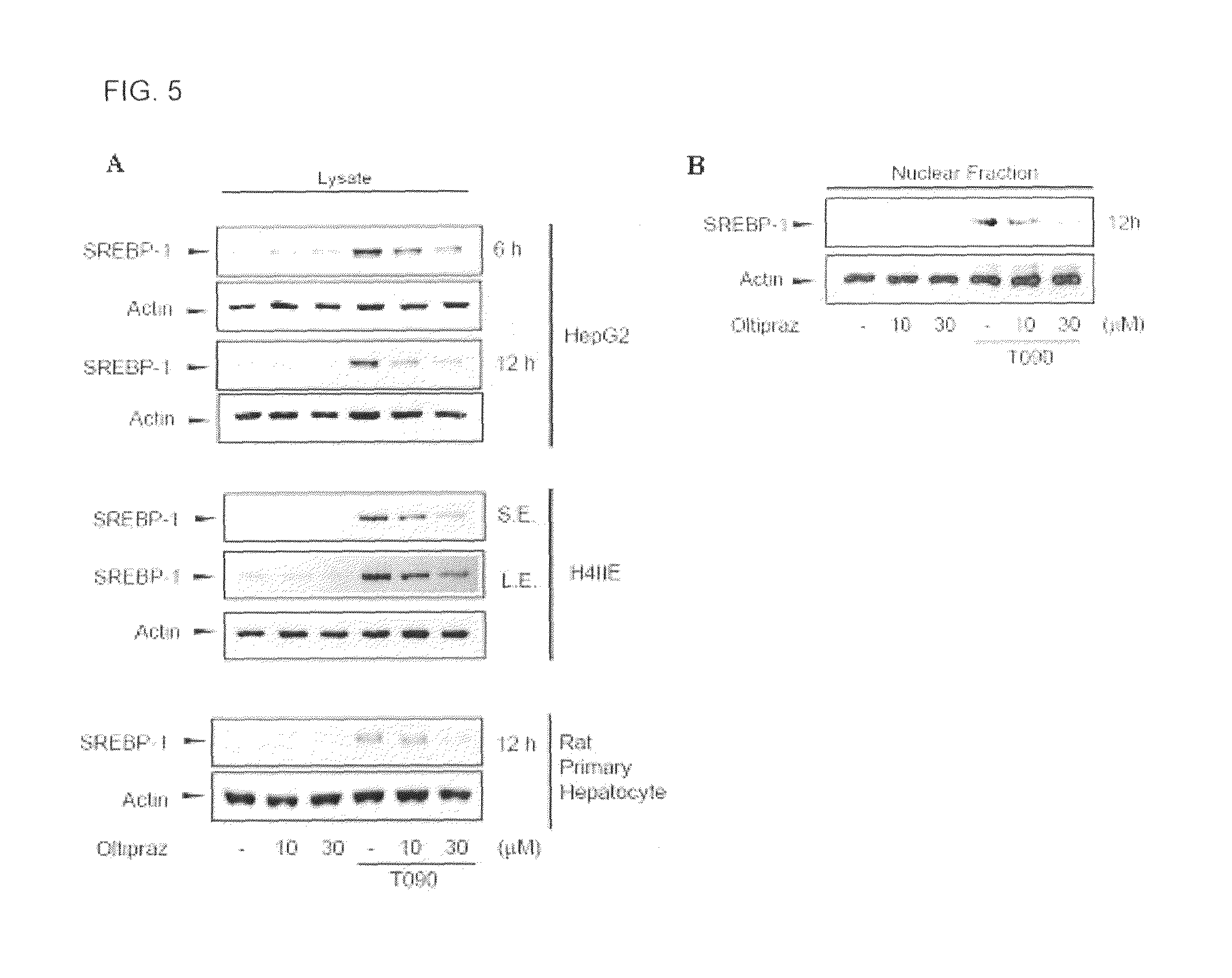
Pharmaceutical composition containing 1,2-dithiolthione derivative for preventing or treating disease caused by overexpression of LXR-α
ActiveUS9370504B2Preventing and treating liver steatosisPreventing andOrganic active ingredientsOrganic chemistryDiseaseX-linked adrenoleukodystrophy
Provided is a pharmaceutical composition that contains a 1,2-dithiolthionederivative, and is effective to prevent and treat a disease caused by overactivity of a liver X receptorα (LXRα) or a sterol response element binding protein (SREBP-1). Specifically, the pharmaceutical composition includes 1,2-dithiolthione derivatives such as 4-methyl-5-(2-pyrazinyl)-1,2-dithiol-3-thione, 3-methyl-1,2-dithiol-3-thione, or 5-(6-methoxypyrazinyl)-4-methyl-1,2-dithiol-3-thione. The pharmaceutical composition is effective for preventing and treating hypertension caused by renin, aldosteronism, adrenoleukodystrophy, glomerulosclerosis, proteinuria, nephropathy, liver steatosis, hypertriglyceridemia or hyperreninemia.
Owner:SEOUL NAT UNIV R&DB FOUND
Non-Anilinic Derivatives of Isothiazol-3(2H)-one 1,1-Dioxides as Liver X Receptor Modulators
The present invention relates to certain novel compounds of the formula (I)to processes for preparing such compounds, to their the utility in modulation of nuclear hormone receptors Liver X Receptor (LXR) α (NR1H3) and / or β (NR1H2) and in treating and / or preventing clinical conditions including cardiovascular diseases such as atherosclerosis; inflammatory diseases, Alzheimer's disease, lipid disorders (dyslipidemias) whether or not associated with insulin resistance, type 2 diabetes and other manifestations of the metabolic syndrome, to methods for their therapeutic use and to pharmaceutical compositions containing them.
Owner:ASTRAZENECA AB
Liver x receptors (LXR) modulators
The present invention relates to sulfonamide-, sulfinamide- or sulfonimidamide containing compounds which bind to the liver X receptor (LXRalpha and / or LXRbeta) and act preferably as inverse agonistsof LXR.
Owner:PHENEX FXR GMBH
Application of liver X receptor activator in anti-nerve inflammatory reaction
InactiveCN102920689AReduce concentrationSmall improvementNervous disorderAmide active ingredientsSide effectSystemic analysis
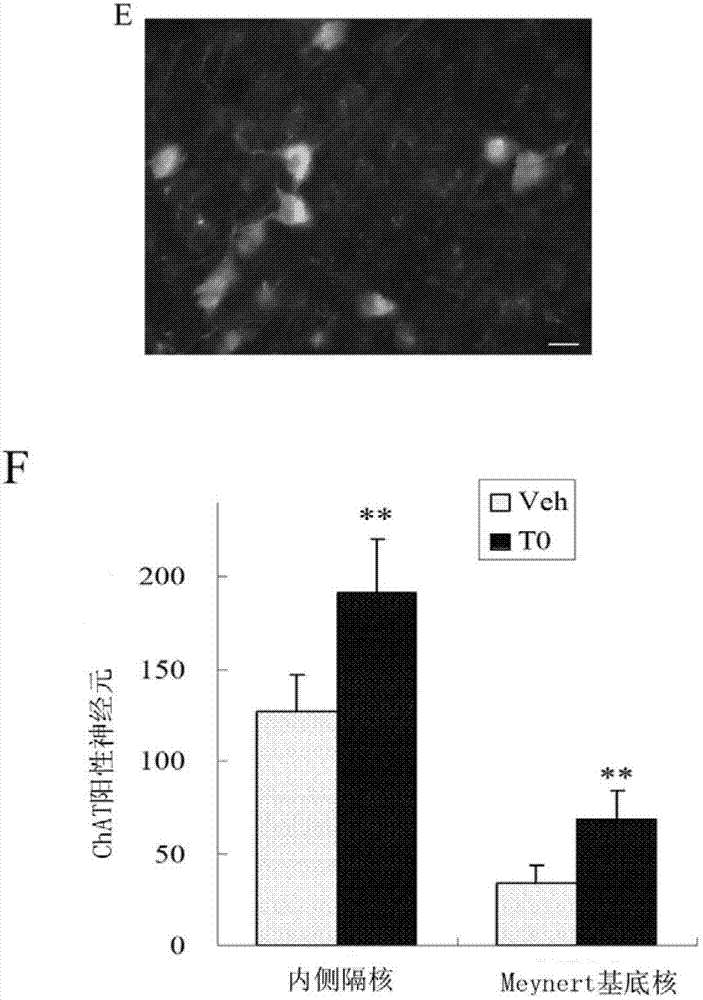
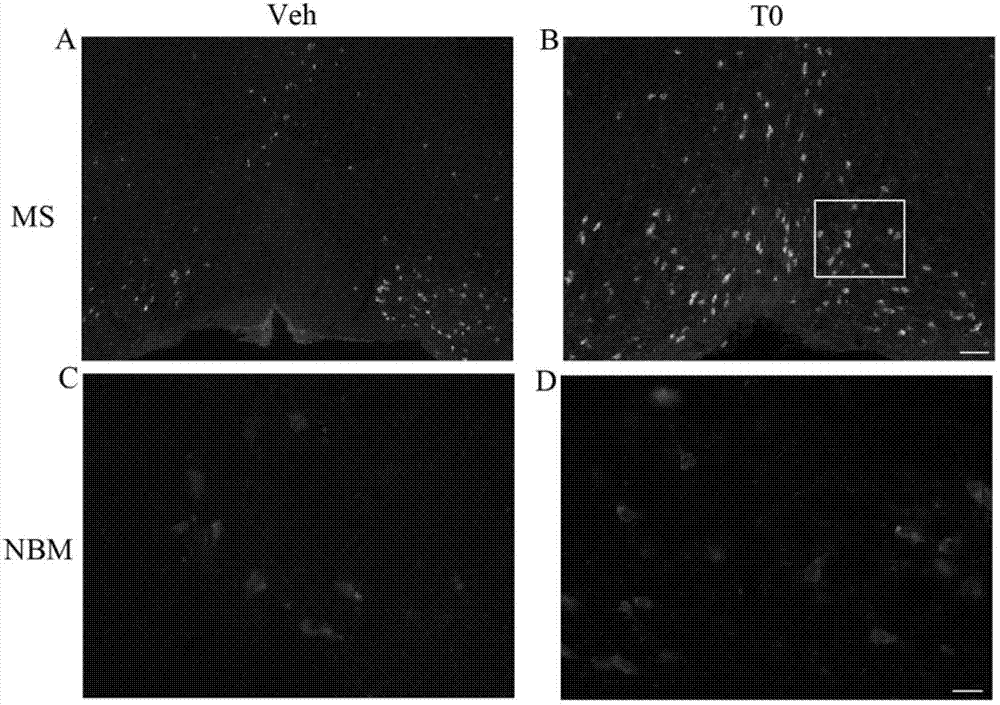
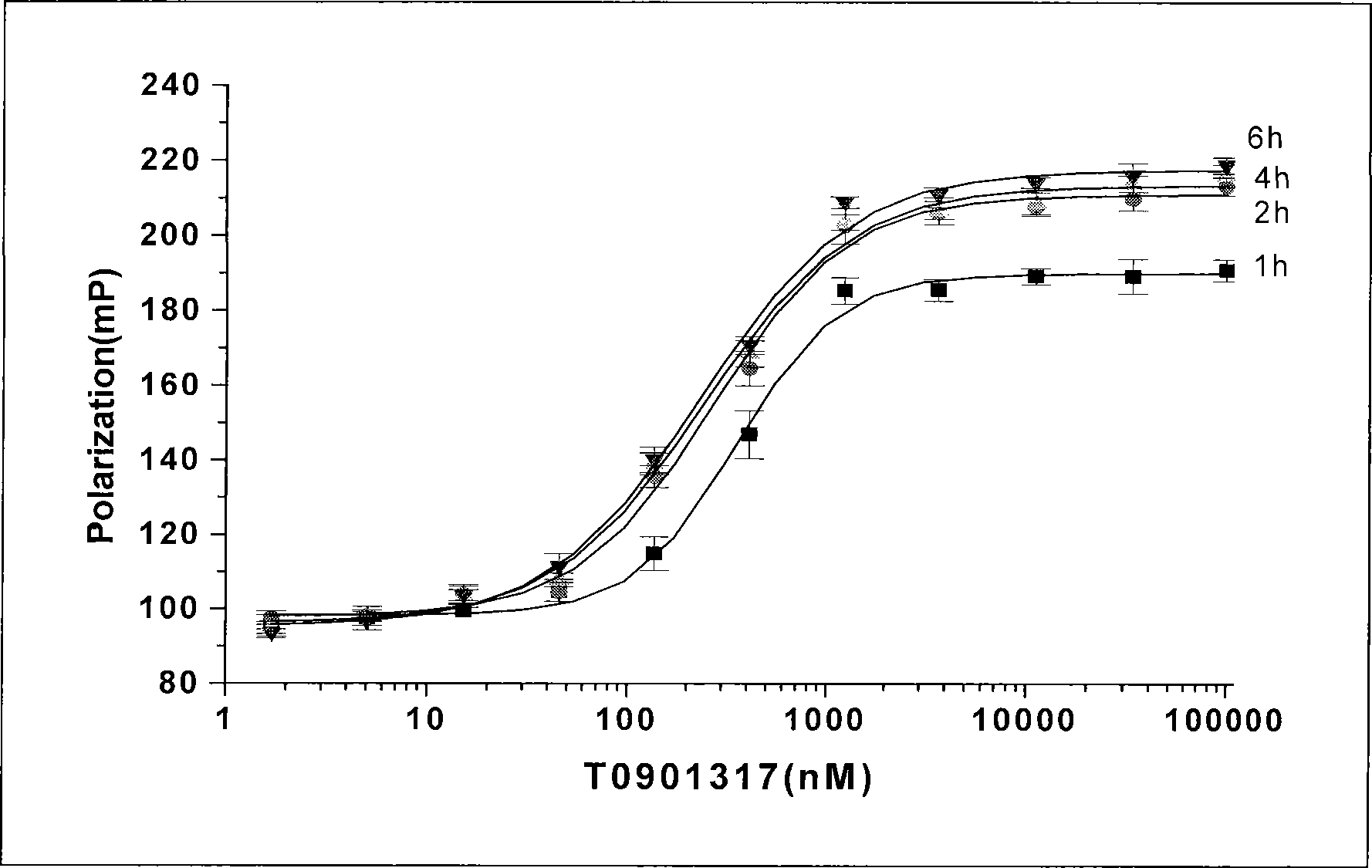
The invention provides application of a liver X receptor activator in anti-nerve inflammatory reaction. The liver X receptor activator is T0901317. The invention also relates to application of T0901317 in preparation of a medicament for treating senile dementia. The liver X receptor activator T0901317 can directly pass through a blood brain barrier, and has the characteristic of low side effect; and the effect of the anti-nerve inflammatory reaction of the liver X receptor activator is systematically analyzed, the liver X receptor activator is expected to be used as a specific medicament for clinically treating the intra-cephalic inflammatory reaction of the senile dementia, and experimental data are provided for clinical treatment of the senile dementia.
Owner:XINXIANG MEDICAL UNIV
Substituted benzamide liver X receptor (LXR) agonists and application
The invention relates to a group of novel compounds serving as liver X receptor (LXR) agonists and a drug composition containing the compounds or medicinal salts thereof and also relates to methods for preparing the compounds and the composition. The invention discloses an application of the compounds as pharmacologically active substances, especially the important application to treatment of cardiovascular diseases such as atherosclerosis, hypertension, hyperlipidemia, hypercholesteremia and coronary heart diseases, metabolic syndromes such as obesity and diabetes mellitus, neurodegenerative diseases such as Alzheimer, immunity-related diseases and the like.
Owner:MEDICINE & BIOENG INST OF CHINESE ACAD OF MEDICAL SCI
Application of saringosterol
The invention discloses an application of saringosterol to preparation of an LXR (liver X receptor) excitant, and particularly discloses an application of 24(S)-saringosterol as an LXR beta excitant.
Owner:OCEAN UNIV OF CHINA
Pyrrole-2,5-dione derivatives as liver x receptor modulators
The present invention relates to certain novel compounds of the Formula I to processes for preparing such compounds, to their the utility in modulation of nuclear hormone receptors Liver X Receptor (LXR) α (NR1H3) and / or β (NR1H2) and in treating and / or preventing clinical conditions including cardiovascular diseases such as atherosclerosis; inflammatory diseases, Alzheimer's disease, lipid disorders (dyslipidemias) whether or not associated with insulin resistance, type 2 diabetes and other manifestations of the metabolic syndrome, to methods for their therapeutic use and to pharmaceutical compositions containing them.
Owner:ASTRAZENECA AB
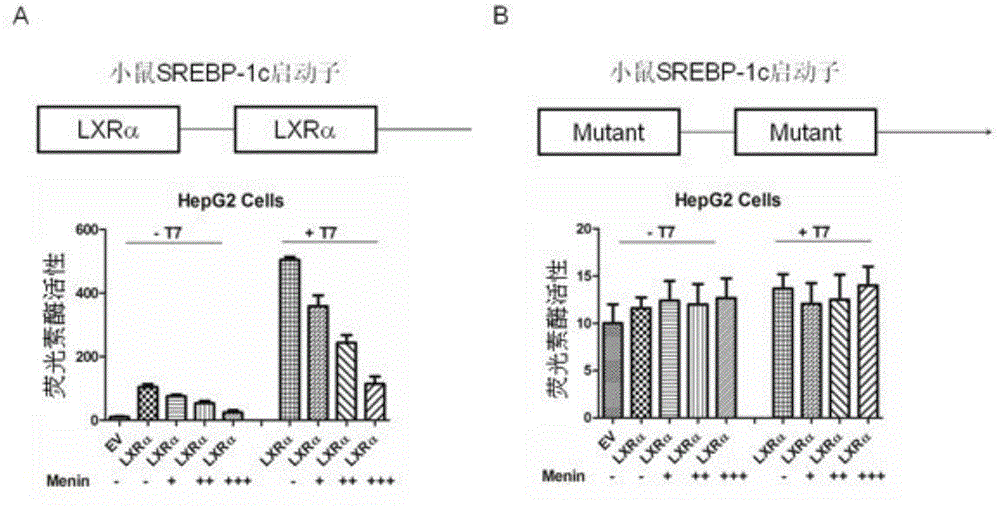
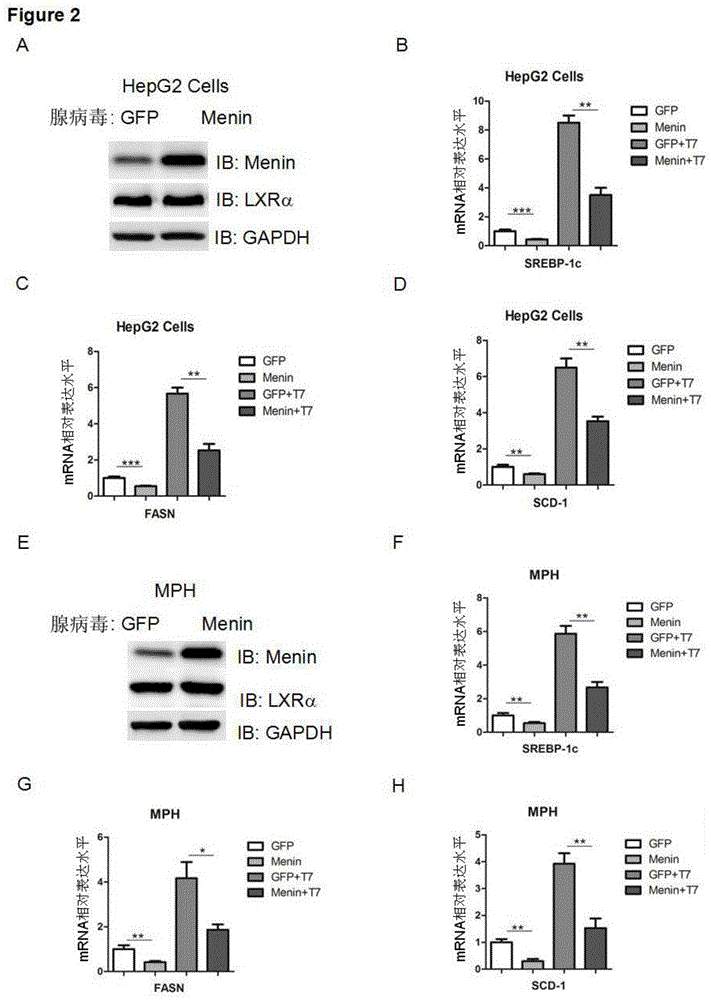
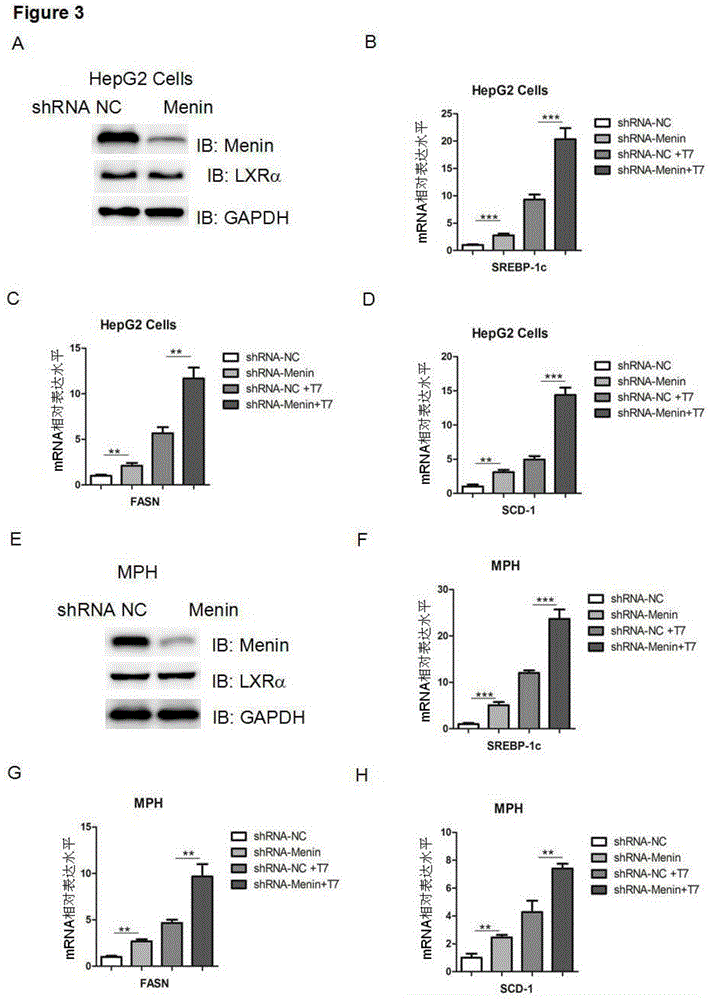
Applications of tumor-inhibiting factor Menin
ActiveCN104593329AGenetic engineeringFermentationFatty liverSterol Regulatory Element Binding Protein 1c
The invention aims to provide an application of Menin in reduction of expression of an endogenous sterol regulatory element-binding protein 1c in HEK293T cells and liver cells, and is characterized by comprising a Menin over-expression step. Another objective of the invention is to provide an application of the Menin in inhibition of transcriptional activity of an HEK293T cell and liver X receptor LXR alpha, and is characterized by comprising a Menin over-expression step. Another objective of the invention is to provide an application of the Menin in inhibition of expression of a target gene of the HEK293T cell and liver X receptor LXR alpha, and is characterized by comprising steps of Menin over-expression and combination with the LXR alpha. According to the applications of the Menin, new research methods and reagents are provided for research and treatment of fatty liver, and a new way is provided for future treatment of fatty liver.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
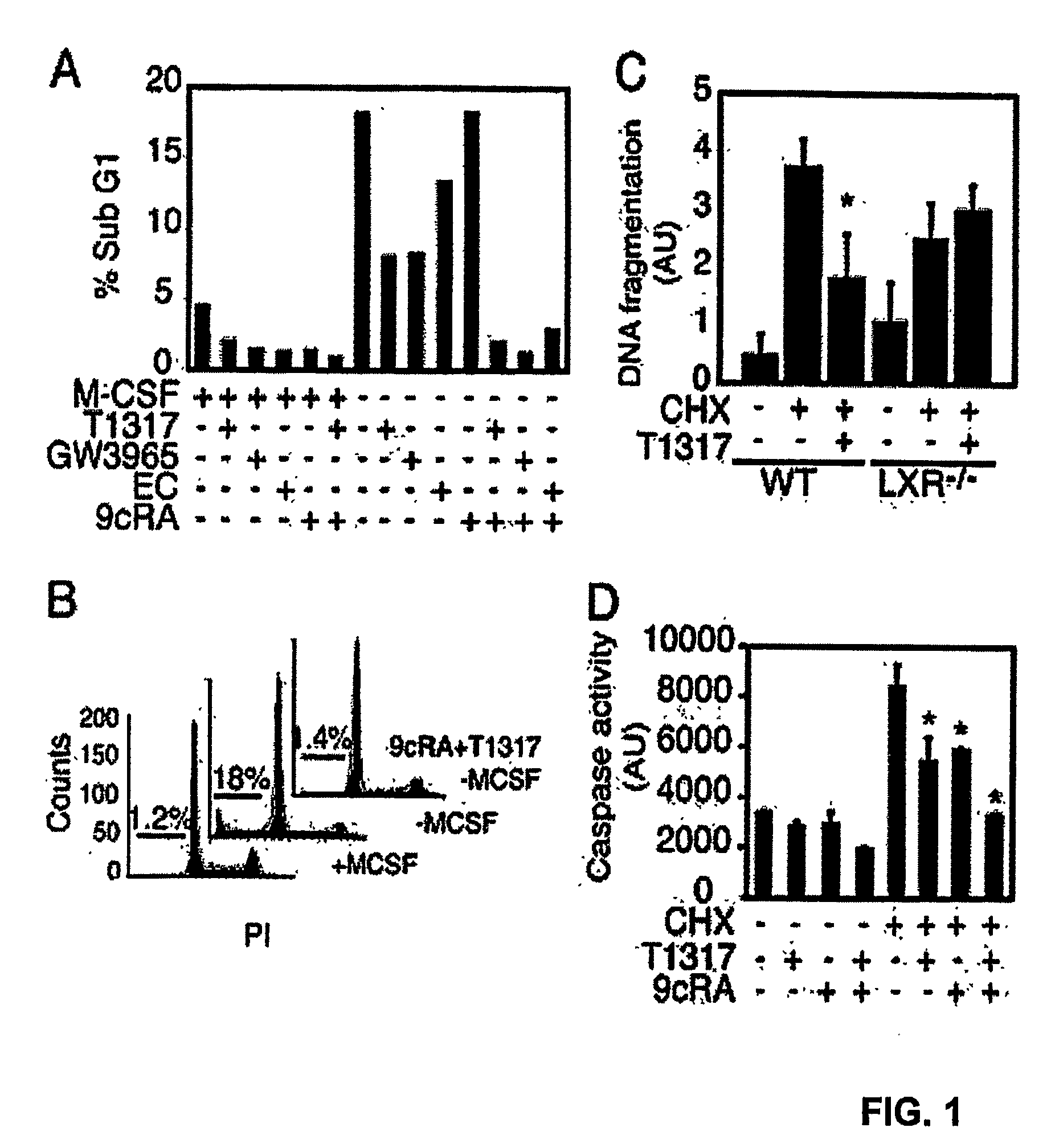
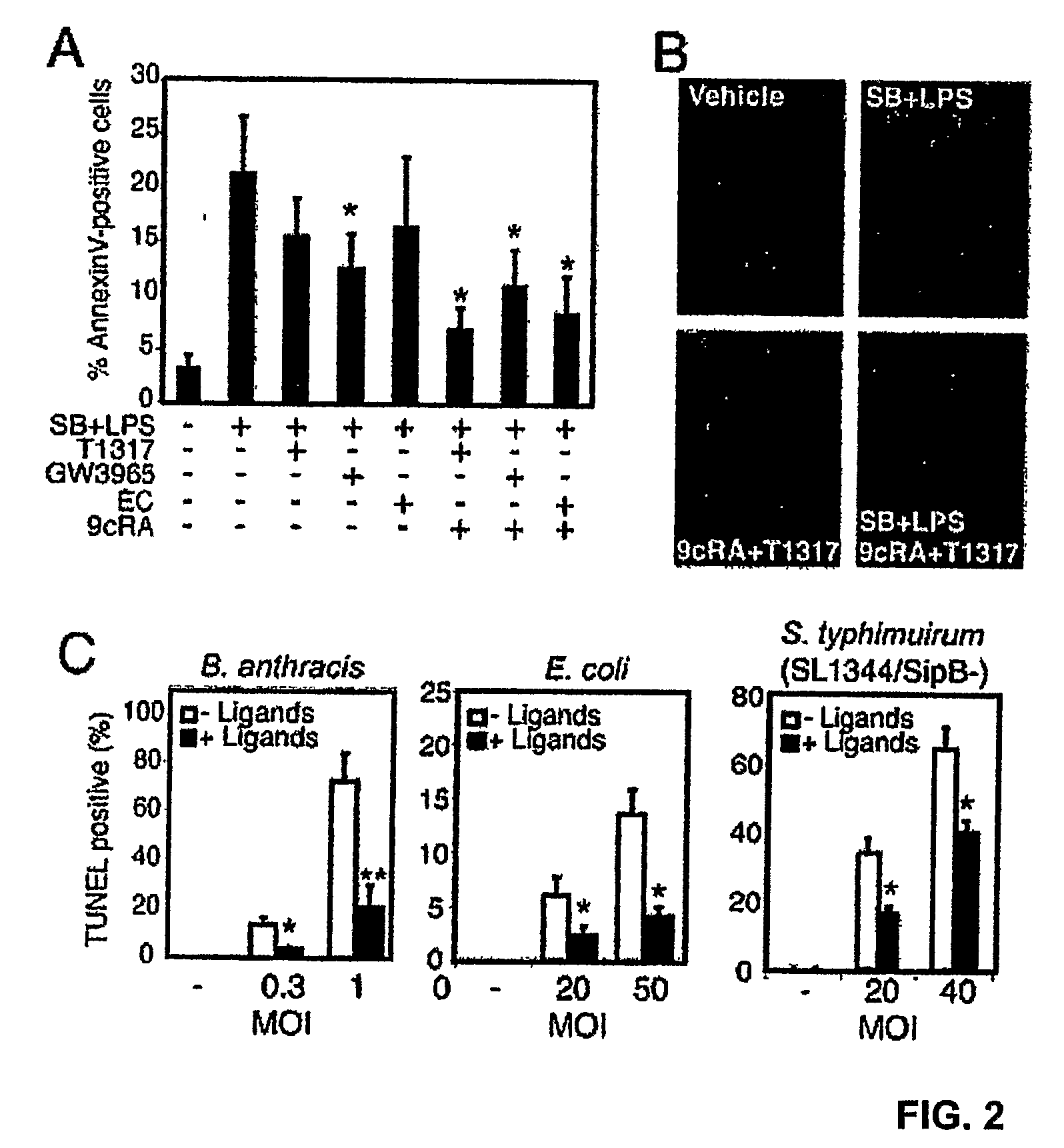
Compounds that Prevent Macrophage Apoptosis and Uses Thereof
The present invention relates to microbial infection, and in particular, the reduction of apoptosis associated with microbial infection, the screening of Liver X Receptor agonist and / or Retinoid X Receptor agonist that reduce apoptosis, and the treatment and analysis of microbial infection in vivo. In one embodiment, the present invention relates to Liver X Receptor agonist and / or Retinoid X Receptor agonist including but not limited to an agonist increasing the activity of Liver X Receptor and / or Retinoid X Receptor.
Owner:RGT UNIV OF CALIFORNIA
Lxr modulators
Owner:EXELIXIS INC
Amine or (THIO)amide containing lxr modulators
PendingCN110914248AAvoid destructionAvoid transshipmentOrganic active ingredientsOrganic chemistryThio-Perylene derivatives
The present invention relates to derivatives of formula (I) which bind to the liver X receptor (LXR[alpha] and / or LXR[beta]) and act preferably as inverse agonists of LXR.
Owner:PHENEX FXR GMBH
Imidazole prodrug LXR modulators
Imidazole prodrugs, pharmaceutically acceptable salts, or isomers thereof, of the invention are disclosed, which are useful as modulators of the activity of liver X receptors (LXR). Pharmaceutical compositions containing the compounds and methods of using the compounds are also disclosed.
Owner:EXELIXIS INC
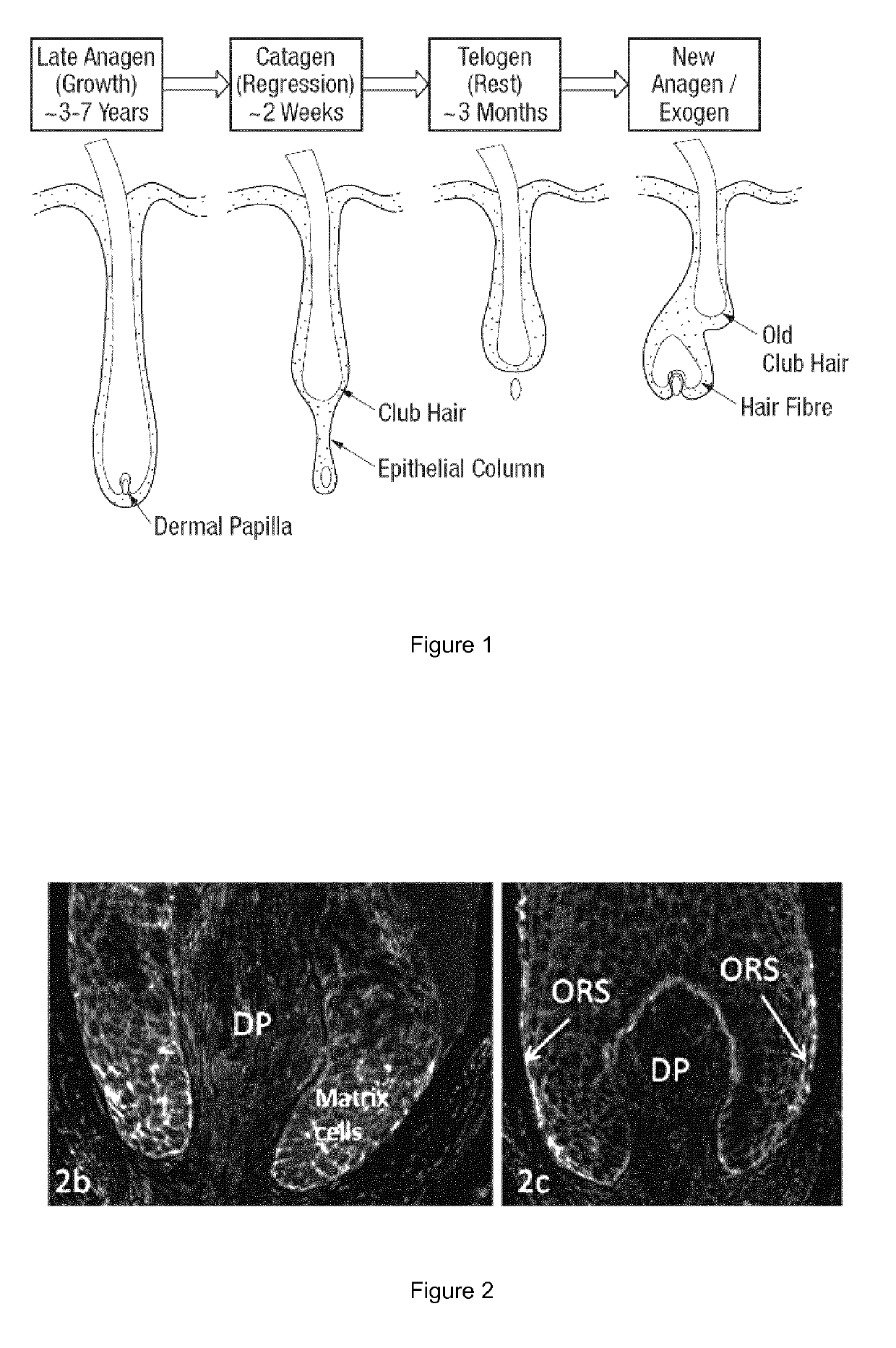
Hair composition
ActiveUS10456344B2Promote growthEffective anti ageingOrganic active ingredientsCosmetic preparationsEpoxyFiber
Disclosed is an oral or topical composition comprising a nuclear factor erythroid-2 related factor 2 agonist and a liver X receptor agonist, wherein the amounts of each of the nuclear factor erythroid-2 related factor 2 agonist and the liver X receptor agonist produce a synergistic benefit of hair fiber growth, wherein the oral or topical composition comprises ≤9, preferably ≤8% w / w β-sitosterol, wherein when the oral or topical composition comprises a catechin, the oral or topical composition comprises 0.001 to 90, preferably 0.005 to 70, most preferably 0.01 to 50% w / w catechins, wherein the oral or topical composition excludes pregnenolone, 4, 5-dihydrofuranodiene-6-one, epoxy santamarin, hydroquinone, longistyline, monacolin K, protoanemonin, N-(2,2,2-tri-fluoro-ethyl)-N-[4-(2,2,2-tri-fluoro-1-hydroxy-1-trifluoromethyl-ethyl)-phenyl]-benzenesulfonamide, dihydronepetalactone, iridomyrmecin, and dihydroactinidiolide, wherein when the oral or topical composition comprises guggelsterone and epigallocatechin gallate, the oral or topical composition excludes a guggelsterone to epigallocatechin gallate weight ratio of 1 to 28, and wherein when the oral or topical composition comprises sodium dilauramide glutamide lysine, the oral or topical composition excludes 0.3% w / w sodium dilauramide glutamide lysine.
Owner:CONOPCO INC D B A UNILEVER
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
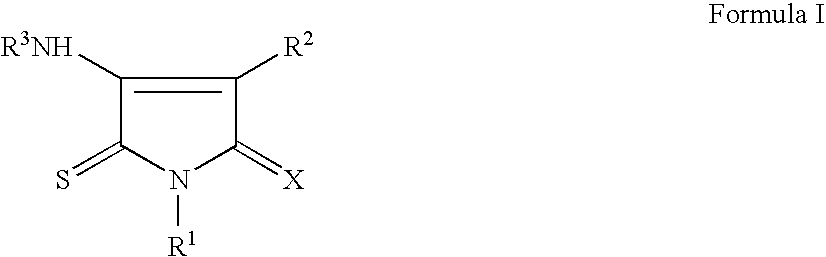
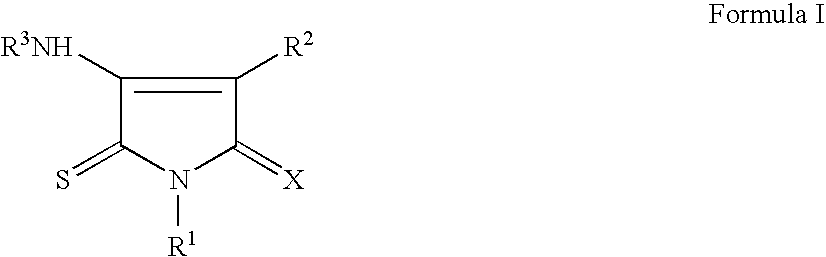
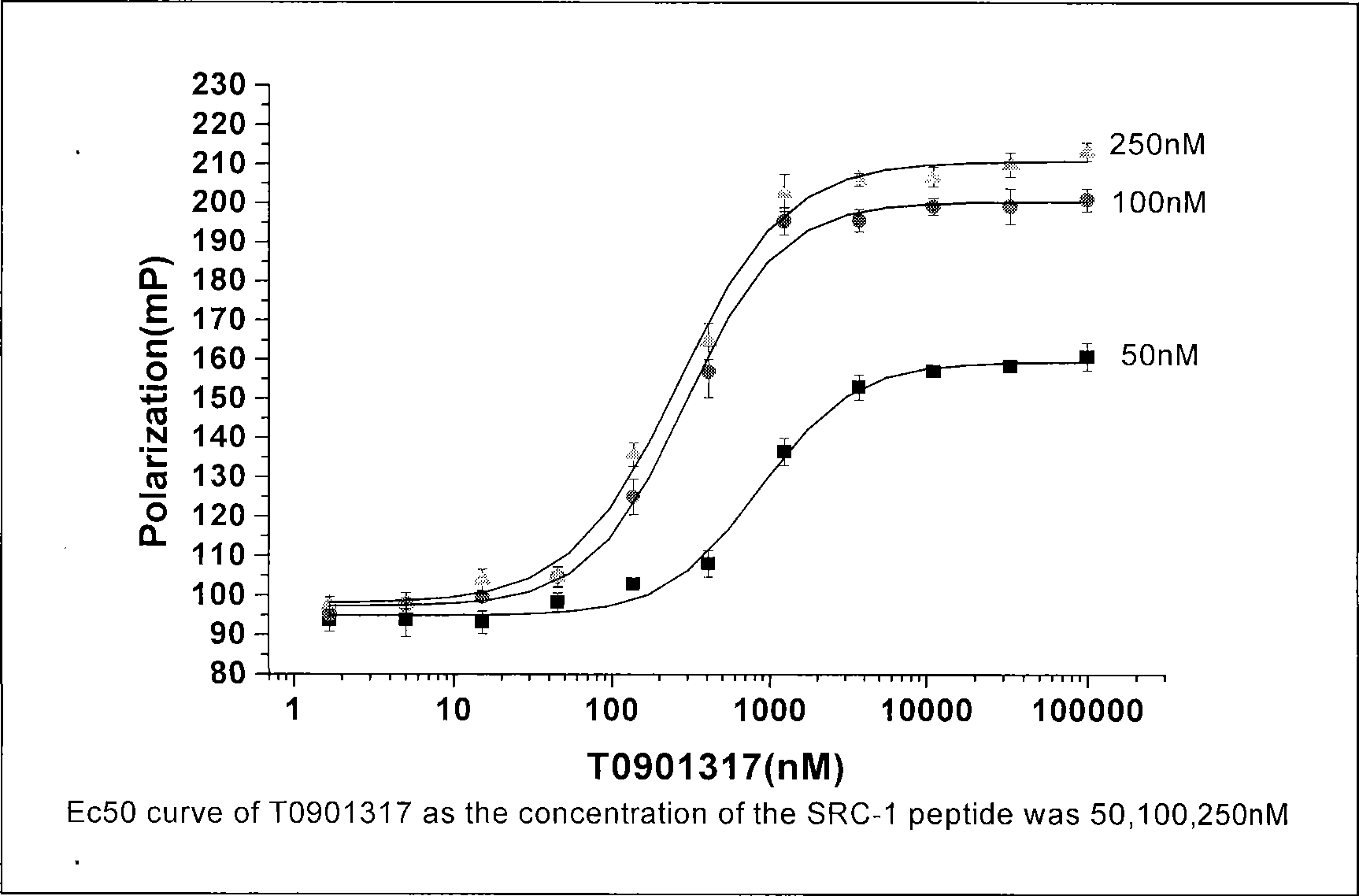
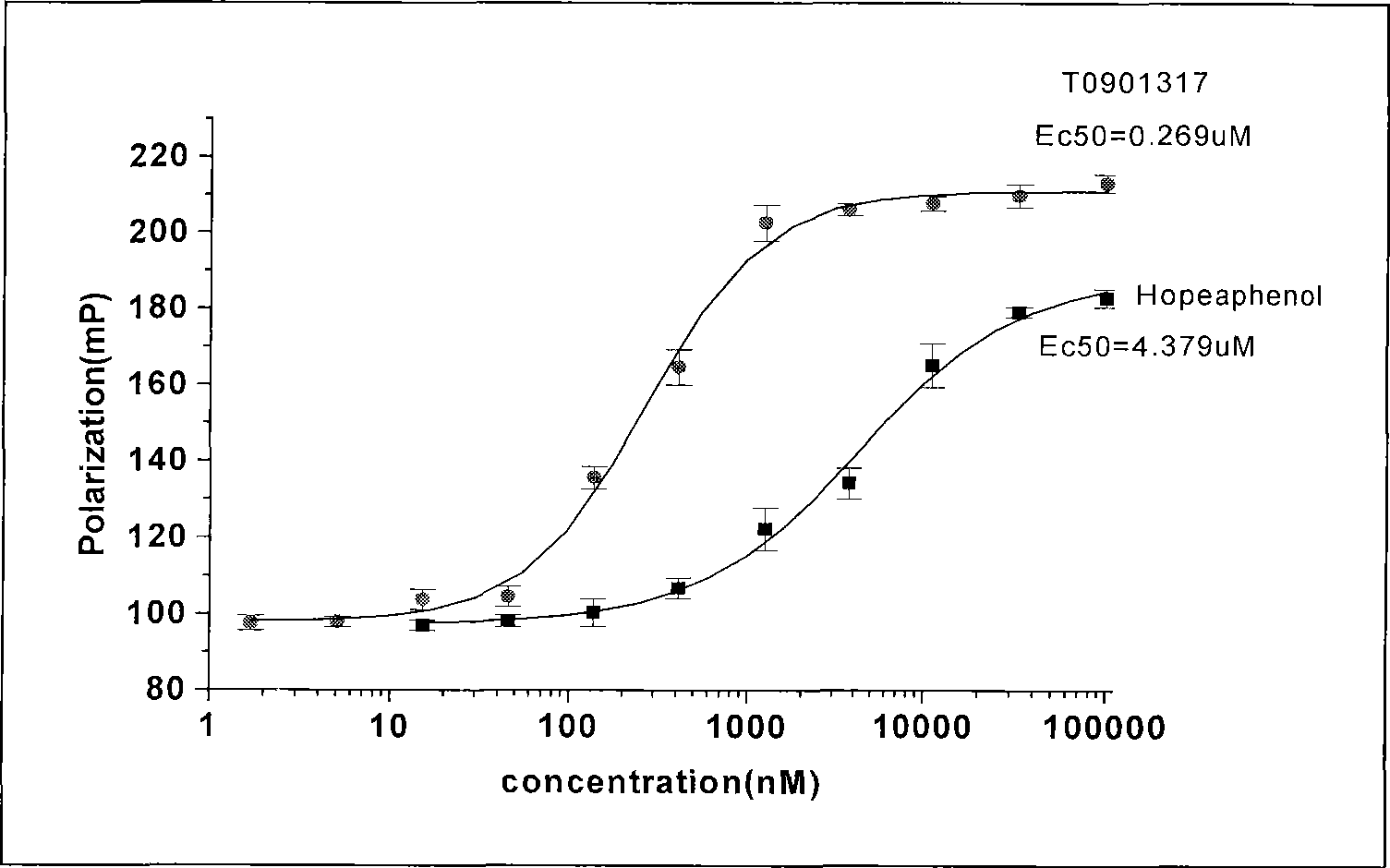
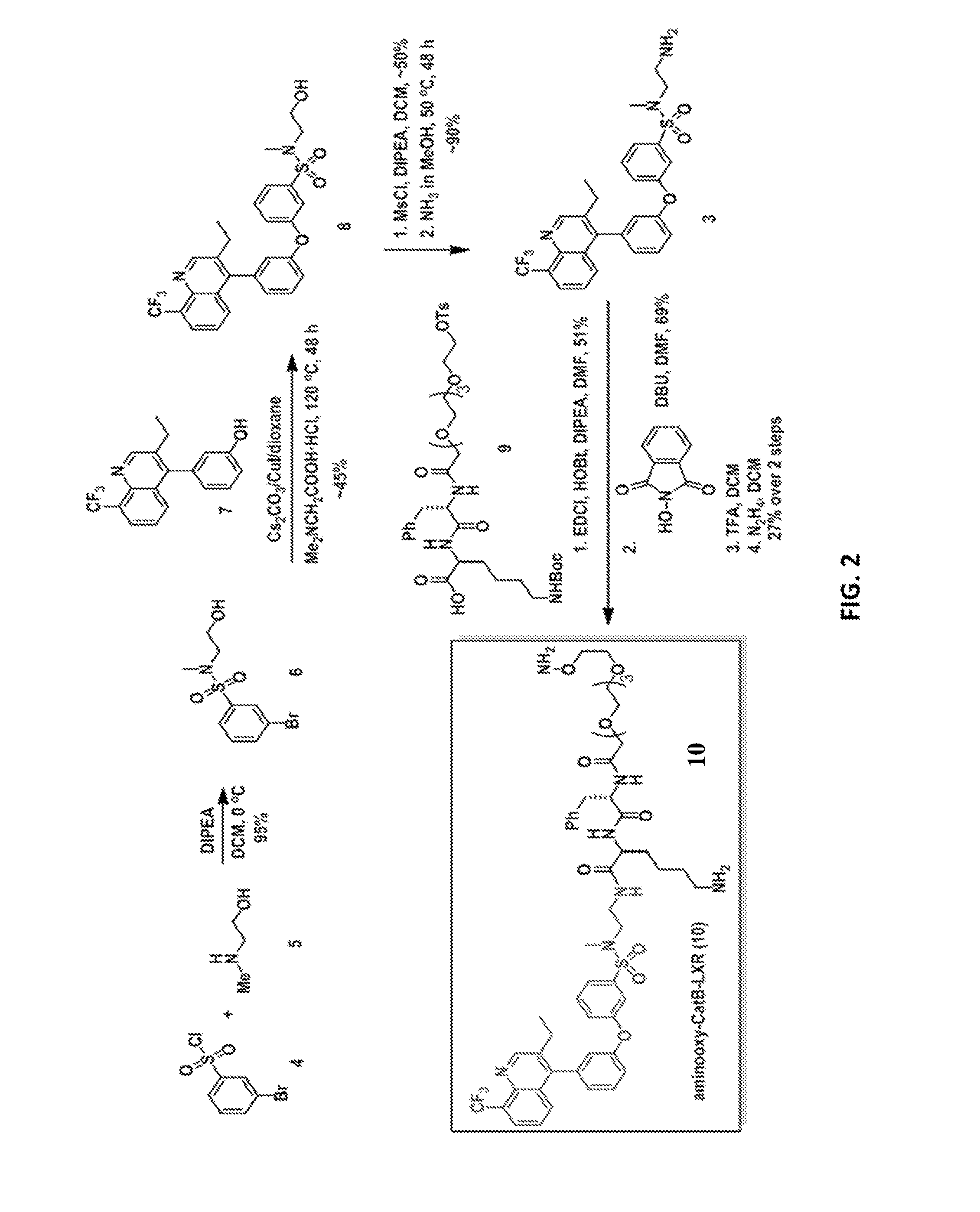
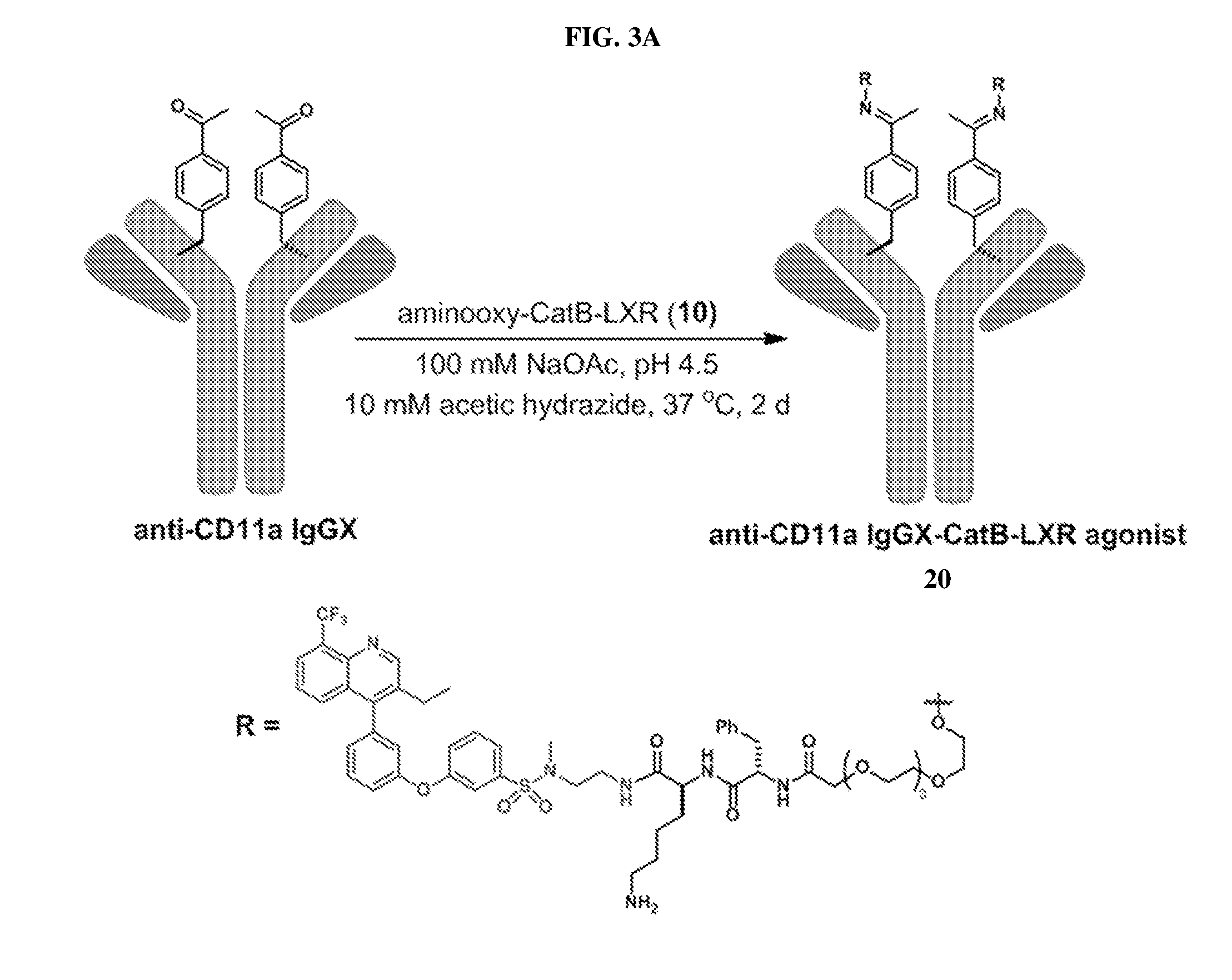
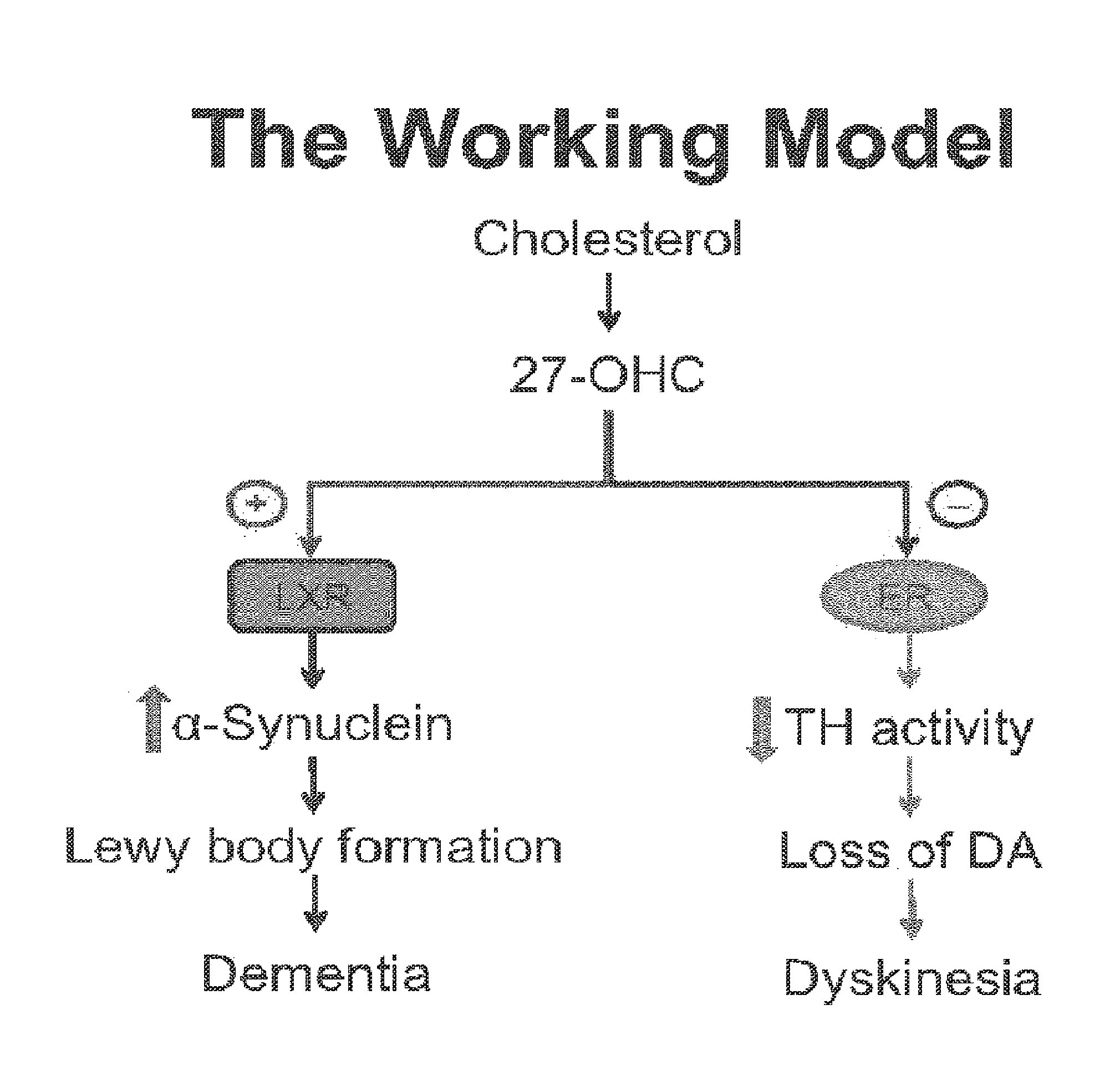
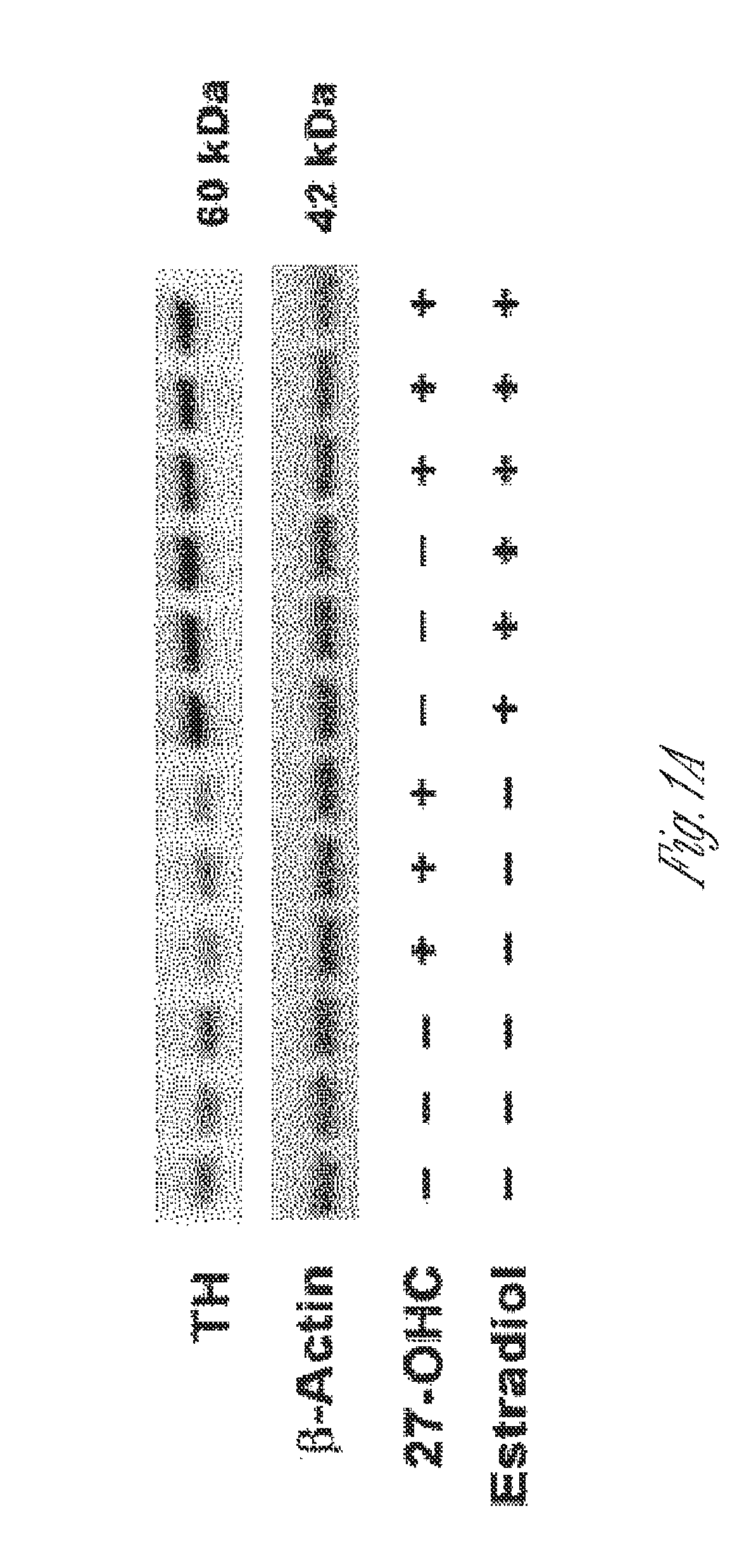
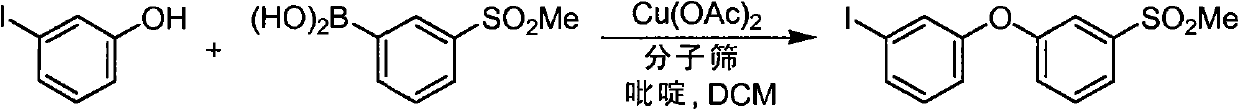
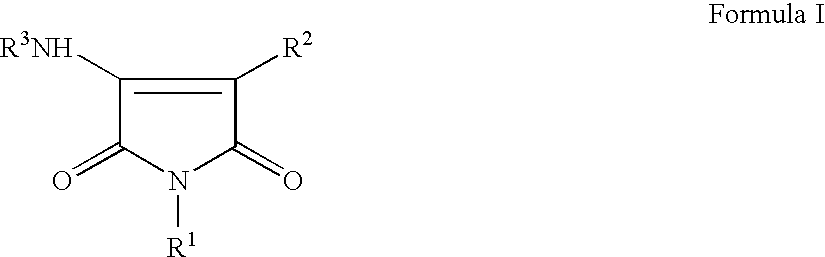
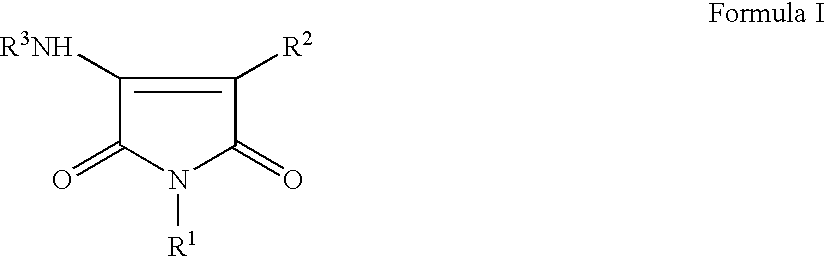
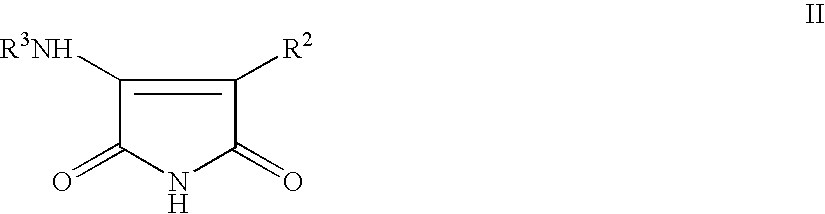
![Pyrazolo [1,5-A] pyrimidine compounds Pyrazolo [1,5-A] pyrimidine compounds](https://images-eureka.patsnap.com/patent_img/263fbbec-6900-487a-bc7f-de0c4d0412cc/000001.png)
![Pyrazolo [1,5-A] pyrimidine compounds Pyrazolo [1,5-A] pyrimidine compounds](https://images-eureka.patsnap.com/patent_img/263fbbec-6900-487a-bc7f-de0c4d0412cc/000002.png)
![Pyrazolo [1,5-A] pyrimidine compounds Pyrazolo [1,5-A] pyrimidine compounds](https://images-eureka.patsnap.com/patent_img/263fbbec-6900-487a-bc7f-de0c4d0412cc/000003.png)