Preparation method of human insulin esters
A technology of human insulin ester and human insulin, applied in the field of preparation of human insulin ester, can solve problems such as low insulin concentration, and achieve the effect of saving cost and improving use efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
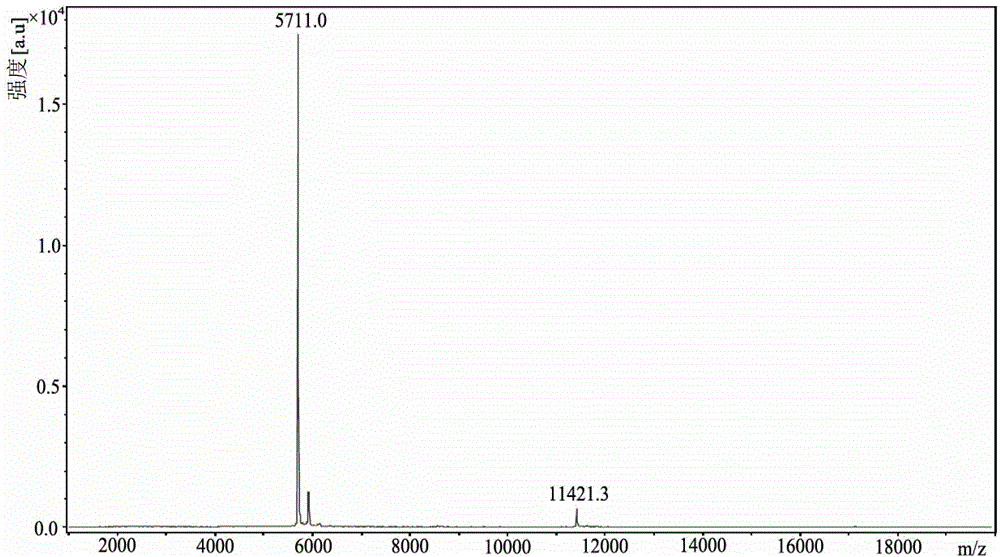
[0033] (1) Take the human proinsulin solution expressed by Pichia pastoris (references: Gao Jiankun, Cai Shaoxi, Fan Kai, etc., containing short C-peptide human proinsulin analog desB 30 Expression and purification in Pichia pastoris, Advances in Biochemistry and Biophysics, 2008, 35 (1): 63-68 "prepared) after macroporous adsorption, reversed-phase chromatography was used to elute with isopropanol, wherein The eluate contains 25% isopropanol by volume, add trypsin according to the mass ratio of proinsulin to trypsin 300:1, stir slowly, and obtain desB-containing 30 The solution of human insulin was adjusted to pH 3.0 with 3M phosphoric acid, and the enzyme cleavage reaction was terminated. desB 30 desB in solution of human insulin 30 The concentration of human insulin is 1.0mM, add the equivalent of desB 30 Zinc chloride equivalent to the molar amount of human insulin, adjusted to pH 5.80 with 5M ammonia water, placed at 4-8°C for 4 hours, then suction-filtered with a 300-...
Embodiment 2
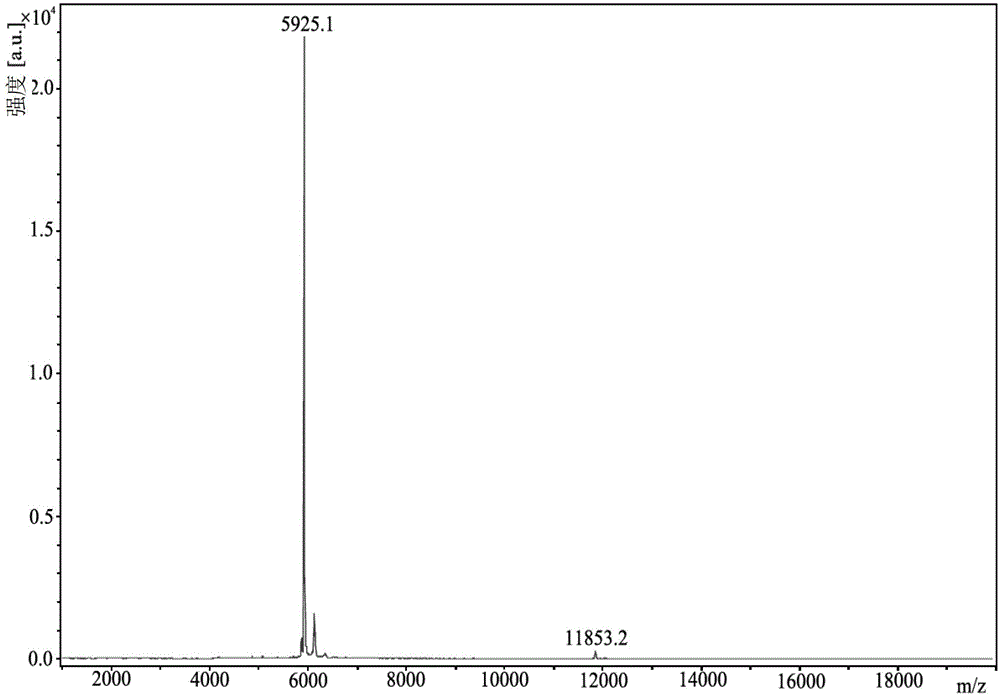
[0038] Dissolving o-tert-butyl ether-l-threonine tert-butyl acetate with desB using dimethyl sulfoxide 30 Human insulin water-containing powder (prepared according to step (1) of Example 1), followed by adding 1,4-butanediol and trypsin aqueous solution (prepared with pure water) to obtain a reaction system, stirred at 25±1°C, and transpeptide reaction 8h, to obtain human insulin ester; wherein, desB 30 The final concentration of human insulin in the reaction system is 17mM, desB 30 Human insulin and o-tert-butyl ether-l-threonine tert-butyl acetate in a molar ratio of 1:15, desB 30 The mass ratio of human insulin and trypsin is 40:1, water (including desB 30 The volume ratio of water contained in human insulin powder and water in trypsin aqueous solution), dimethyl sulfoxide and 1,4-butanediol is 20%:40%:40%.
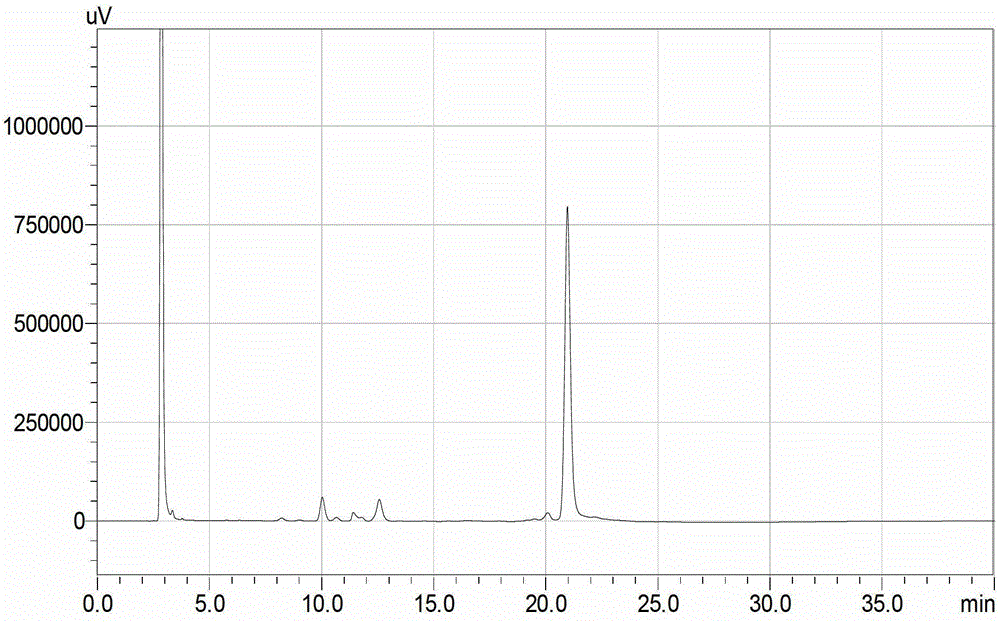
[0039] HPLC detection and analysis detected the transpeptide efficiency at 2, 4, 6, and 8 hours, and the transpeptide efficiency at 2, 4, 6, and 8 hours were 85.52%...
Embodiment 3
[0041] (1) Take the aqueous solution of human insulin pro-insulin refolded after expression in Escherichia coli (references: Weng Liang, Liu Lijun, Wang Zhi, etc. Purification of recombinant human insulin and preliminary identification of its properties. Biotechnology Bulletin, 2003, 14 (5) : 387-389), add trypsin according to the mass ratio of proinsulin to trypsin 300:1, stir slowly, the solution after enzymatic digestion at 37℃, in which desB 30 The concentration of human insulin is 0.5mM according to desB 30 The molar ratio of human insulin: zinc chloride is 1:1.5, add zinc chloride, adjust the pH to 5.60 with 3M phosphoric acid, precipitate at 4-8°C for 4 hours, and obtain desB by suction filtration 30 Human insulin water-containing powder, its water content is 45% as measured by a moisture analyzer, and its content is detected by HPLC.
[0042] (2) Use dimethyl sulfoxide to dissolve o-tert-butyl ether-l-threonine tert-butyl ester hydrochloride (H-Thr(tBu)-OtBu.HCl) and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com