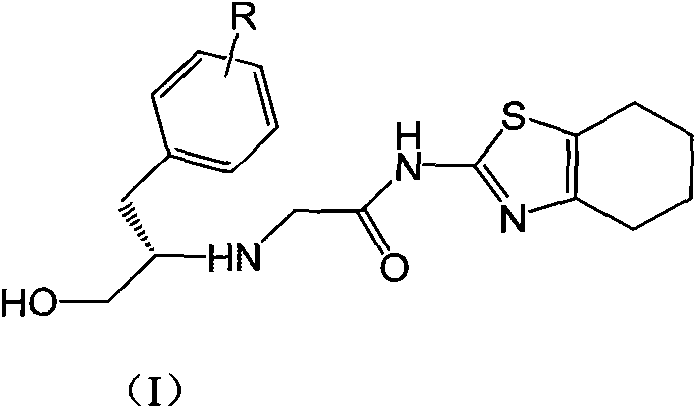
Benzyl-substituted thiazolocyclohexane compounds, and preparation methods and antitumor uses thereof
A compound and hydroxyl technology, applied in organic chemistry, drug combination, antineoplastic drugs, etc., can solve problems such as poor selectivity, low specificity, and multidrug resistance of cancer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024]
[0025] Reaction raw materials: commercially available or self-made.
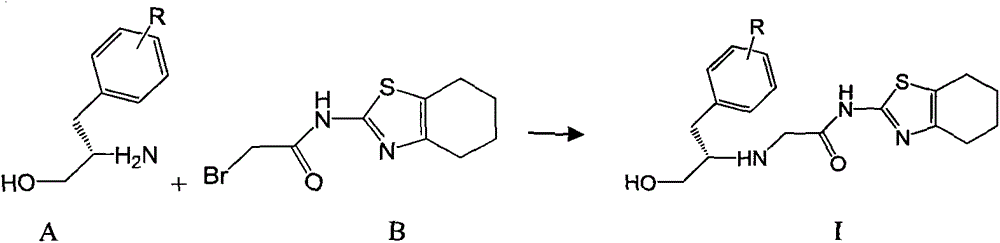
[0026] 1.67g (10mmol) of compound A-1 and 2.75g (10mmol) of compound B-1 were dissolved in 20mL of dry THF, and then 3.04g (30mmol) of Et 3 N, then stirred at room temperature until the reaction was complete (24-48 hours). The reaction mixture was poured into ice water, extracted with 50 mL×3 dichloromethane, the combined organic phases were washed with brine, dried over anhydrous sodium sulfate, and the solvent was evaporated on a rotary evaporator to obtain the crude product of I-1, which was subjected to column chromatography Purification gave the pure product of I-1, white solid, mp.185-187°C; MS, m / z=362 ([M+H] + ).
Embodiment 2
[0028]
[0029] 1.67g (10mmol) of compound A-1 and 2.75g (10mmol) of compound B-1 were dissolved in 20mL of dry THF, then 3.88g (30mmol) of DIPEA was added, and then refluxed with stirring until the reaction was complete (within 10 hours). The reaction mixture was poured into ice water, extracted with 50 mL×3 dichloromethane, the combined organic phases were washed with brine, dried over anhydrous sodium sulfate, and the solvent was evaporated on a rotary evaporator to obtain the crude product of I-1, which was subjected to column chromatography Purification gave the pure product of I-1, white solid, mp.185-187°C; MS, m / z=362 ([M+H] + ).
Embodiment 3
[0031]
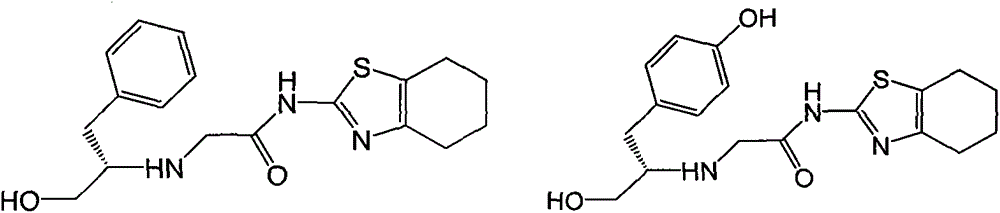
[0032] 1.51g (10mmol) of compound A-1 and 2.75g (10mmol) of compound B-1 were dissolved in 20mL of dry THF, then 3.88g (30mmol) of DIPEA was added, and then refluxed with stirring until the reaction was complete (within 10 hours). The reaction mixture was poured into ice water, extracted with 50mL×3 dichloromethane, the combined organic phases were washed with brine, dried over anhydrous sodium sulfate, and the solvent was evaporated on a rotary evaporator to obtain the crude product of I-2, which was subjected to column chromatography Purification gave the pure product of I-2, white solid, mp.216-219°C; MS, m / z=346 ([M+H] + ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com