Pharmaceutical composition containing L-DNA
A technology of L-NDA and -D-DNA, which is applied in the field of pharmaceutical compositions containing L-DNA, and can solve problems such as unfavorable pharmacokinetics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Embodiment 1: cracking test
[0070] The activity of L-ribozyme and D-ribozyme was measured under various conditions. The basic conditions are as follows. Incubate 0.2 μM target RNA or DNA with 10 μl reaction mixture in 50 mM Tris-HCL buffer, pH 7.5, 20°C for 2 hours in the presence of 2 μM DNase or RNase (so DNase or RNase / target ratio of 10:1). Prior to the reaction, the target RNA or DNA and DNase or RNase were denatured at 72°C for 2 minutes and cooled slowly (1°C / min) to 25°C in a heat seal. Study Mg at concentrations of 0.1-10 mM 2+ - The effect of ions. Lysates were separated by 20% polyacrylamide gel electrophoresis in 0.09 Tris-borate buffer, pH 8.3, in the presence of 7 M urea. Fluorescence analysis was performed on a Phosphoimager Fuji Film FLA 5100. Data were acquired using the program Fuji analysis program. Graphs were made using Excel.
Embodiment 2
[0071] Embodiment 2: the preparation of target sequence and ribozyme
[0072] All target sequences were prepared by chemical synthesis. The purity of the synthesized product is greater than 90%.
[0073] As the DNase or RNase, the variable region of the DNase or RNase is selected around the cleavage site trimer according to the target sequence, and the RNase sequence or DNase sequence is synthesized. The purity of the synthesized product is greater than 85%.
[0074] All synthetic products were labeled with fluorescein at the 5'-end.
Embodiment 3
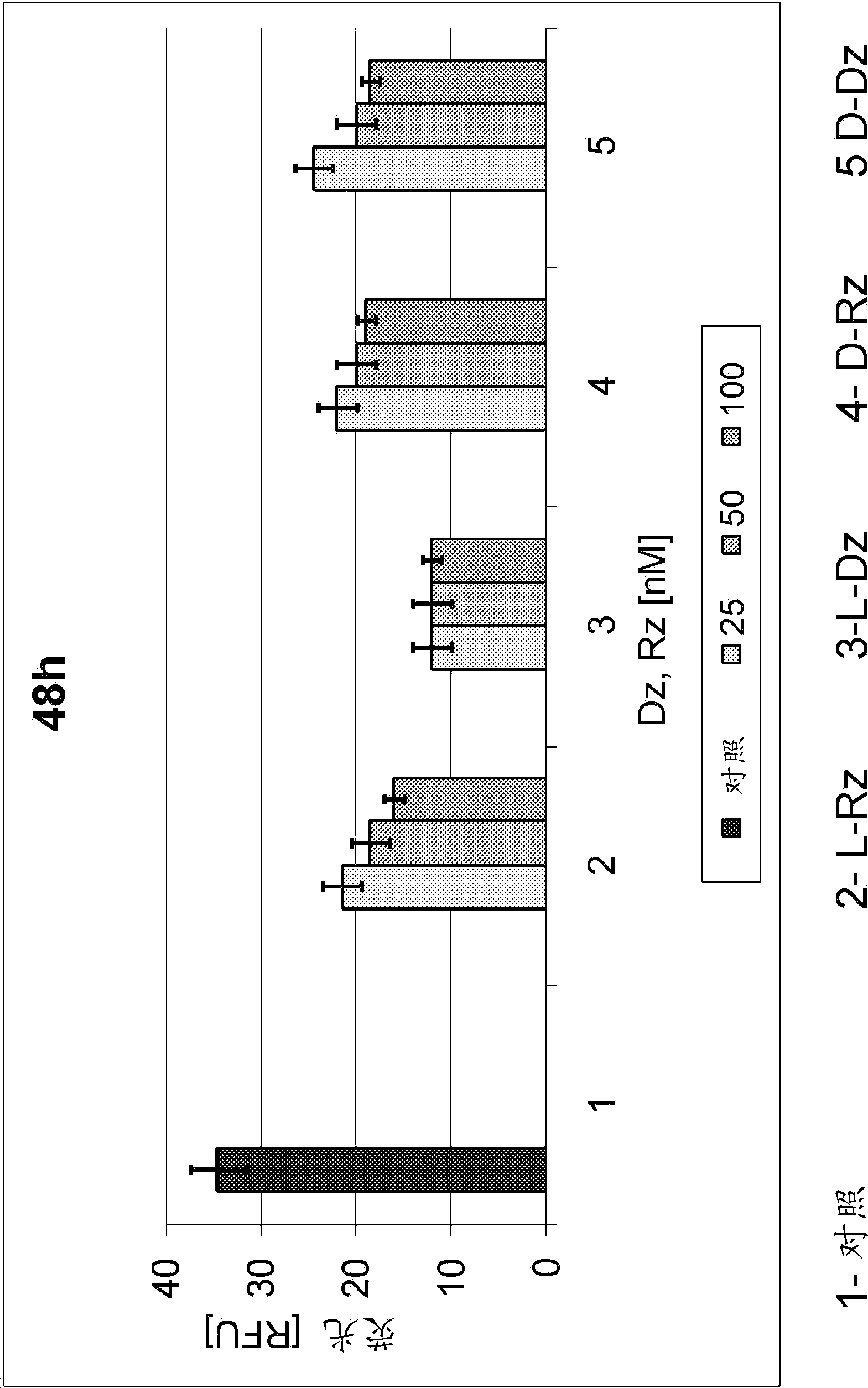
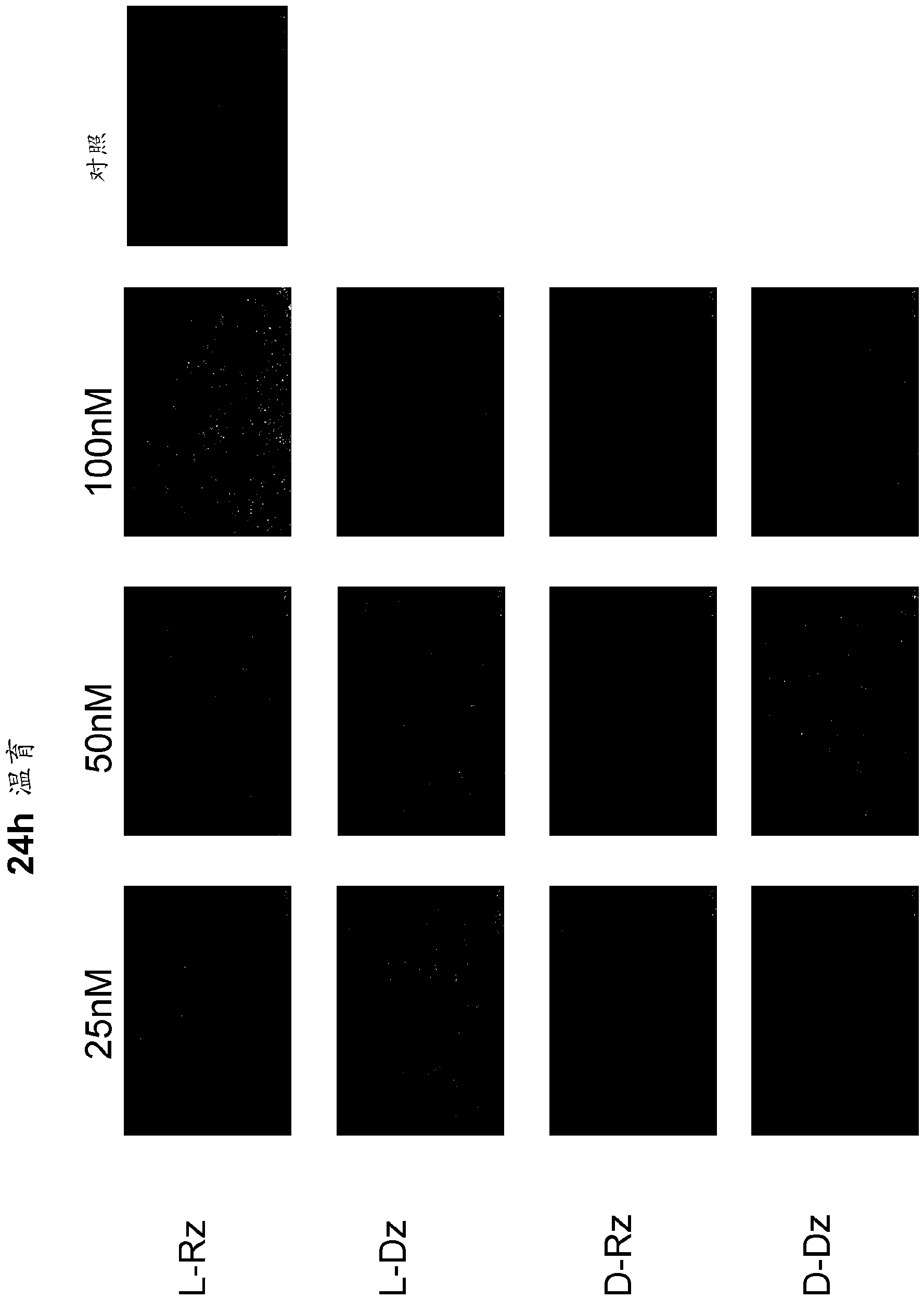
[0075] Example 3: Measuring activity in cells
[0076] According to the protocol, HeLa cells were transfected with 1 μg EGFP plasmid. Incubation was performed with 25, 50 or 100 nM solutions of the DNase or RNase used. After 24 h or 48 h, the cells were analyzed with a Leica microscope, or the fluorescence intensity (RFU) was measured according to the protocol with the aid of Multi-Mode Microplate Reader Synergy-2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com