Combination therapies comprising anti-ERBB 3 agents
A combined therapy and pharmaceutical technology, which is applied in drug combinations, antineoplastic drugs, antibody medical components, etc., can solve problems such as changing bioavailability and affecting safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
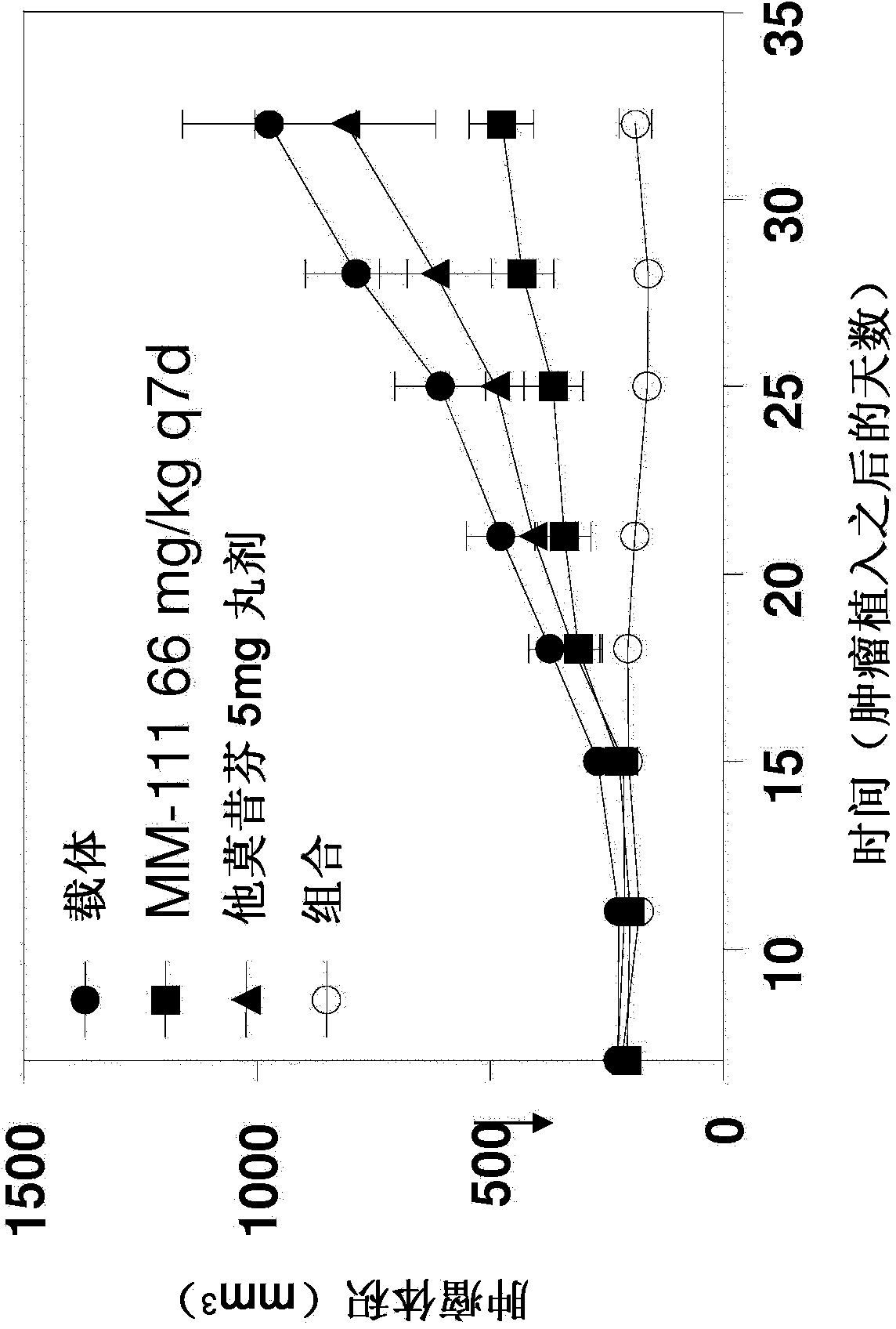
[0063] Example 1: Combination therapy with MM-111 and tamoxifen inhibits tumor growth in vivo.
[0064] To compare the effect of combination therapy with MM-111 and tamoxifen on tumor growth in vivo, estrogen-stimulated mice were prepared in a xenograft model using the methods described above or minor variations thereof. Mice were inoculated with tumor forming BT474-M3 cells and given placebo (vehicle control), MM-111, tamoxifen, or a combination of MM-111 and tamoxifen on day 7 and tumor growth was measured over time . as in figure 1 As shown in , this in vivo BT474-M3 xenograft model showed resistance to tamoxifen treatment, but when the combination of MM-111 and tamoxifen was administered to mice, the combination therapy exhibited significant inhibited tumor growth to a greater extent. For the combination group, statistical significance was observed from day 28 when compared to vehicle control, from day 21 when compared to MM-111, and from day 25 when compared to tamox...
Embodiment 2
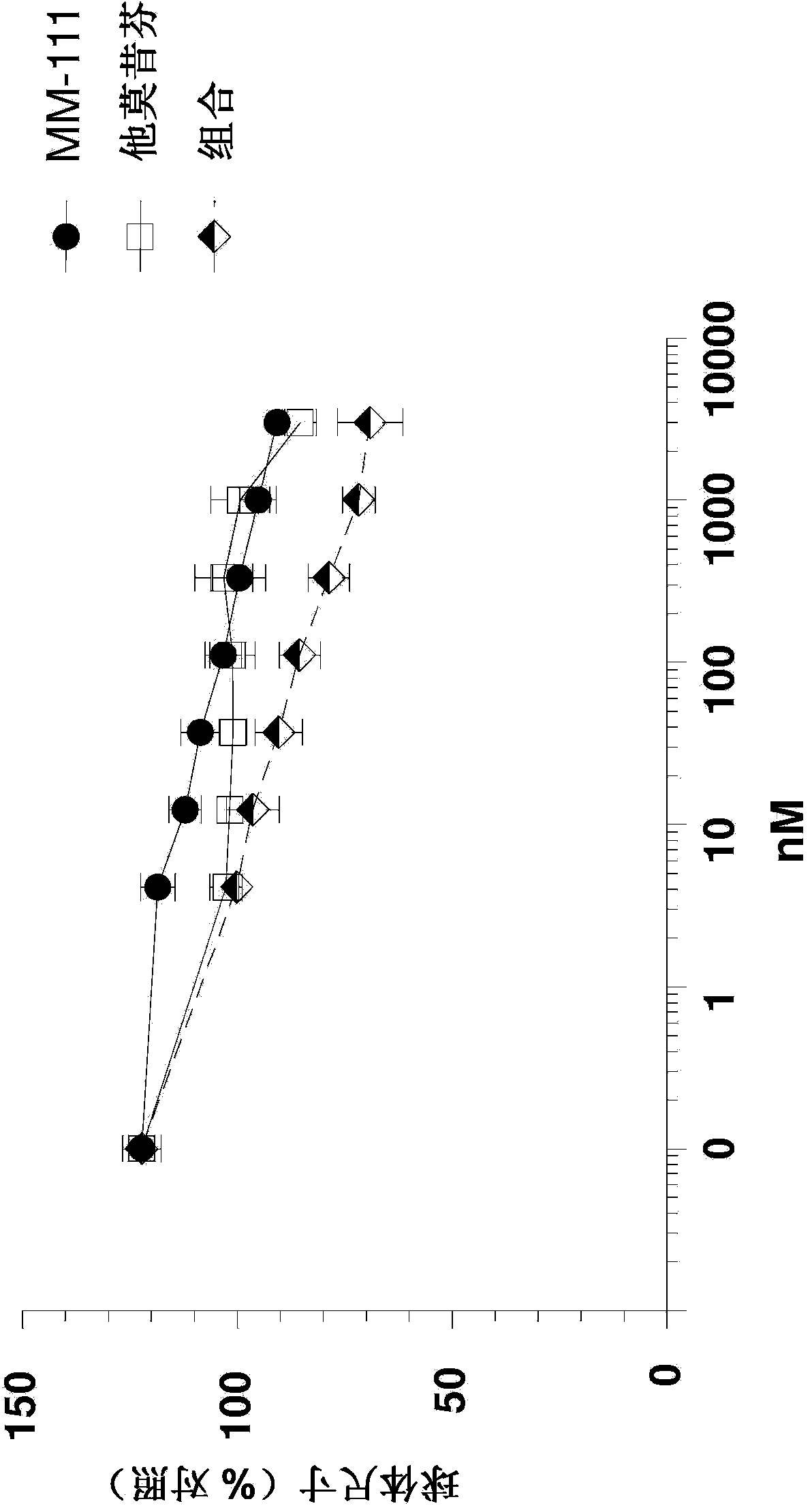
[0065] Example 2: MM-111 Positively Comparable to Antiestrogens in Inhibiting Estrogen-Stimulated Spheroid Growth sexual union
[0066] Use multicellular spheroids to mimic tumor growth and microenvironmental conditions in vitro. To further investigate the ability of MM-111 to inhibit cell growth when MM-111 is combined with antiestrogens, spheroids of BT474-M3 were prepared using the method described above or a slight variation thereof and treated with ErbB2-binding therapeutics and / or anti-estrogen therapy. Use of MM-111, tamoxifen, or a combination of MM-111 and tamoxifen ( Figure 2a ); trastuzumab, tamoxifen, or a combination of trastuzumab and tamoxifen ( Figure 2b ); MM-111, fulvestrant, or a combination of MM-111 and fulvestrant ( Figure 2c ); trastuzumab, fulvestrant, or a combination of trastuzumab and fulvestrant ( Figure 2d ); or MM-111, trastuzumab, or a combination of MM-111 and trastuzumab ( Figure 2e ) range of doses to treat spheroids of estroge...
Embodiment 3
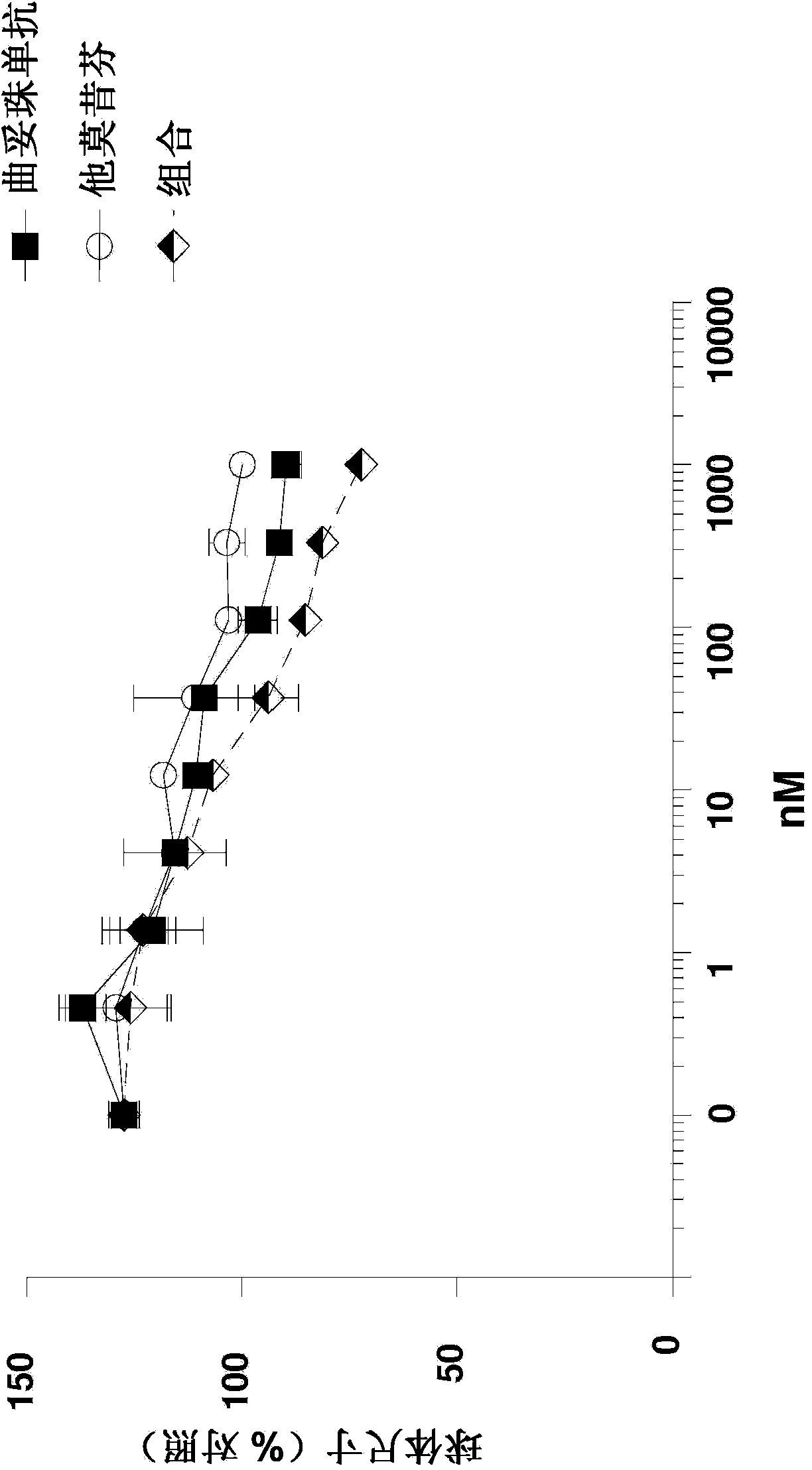
[0067] Example 3: Positivity of MM-111 to Antiestrogens in Inhibiting Heregulin-Stimulated Spheroid Growth sexual union
[0068] To further investigate the ability of MM-111 to inhibit cell growth when combined with antiestrogens, spheroids of heregulin (HRG)-stimulated BT474-M3 cells were prepared using the method described above or a slight variation thereof and MM-111 was used to -111, tamoxifen, or a combination of MMM-111 and tamoxifen ( Figure 3a ); trastuzumab, tamoxifen, or a combination of trastuzumab and tamoxifen ( Figure 3b ); MM-111, fulvestrant, or a combination of MM-111 and fulvestrant ( Figure 3c ); trastuzumab, fulvestrant, or a combination of trastuzumab and fulvestrant ( Figure 3d ); or MM-111, trastuzumab, or a combination of MM-111 and trastuzumab ( Figure 3e ) dose range to treat spheroids. MM-111 inhibited heregulin-induced spheroid growth but tamoxifen ( Figure 3a ),Trastuzumab( Figure 3b ) and fulvestrant ( Figure 3c ) did not inhib...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com