1,3-Substituted-5-Acetamidolindolinone Compounds and Their Application in Antineoplastic Drugs
A technology of acetaminoindolinone and acetamidoindoledione, which is applied in the field of pharmaceutical applications, and achieves the effects of simple operation process, short synthetic route and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
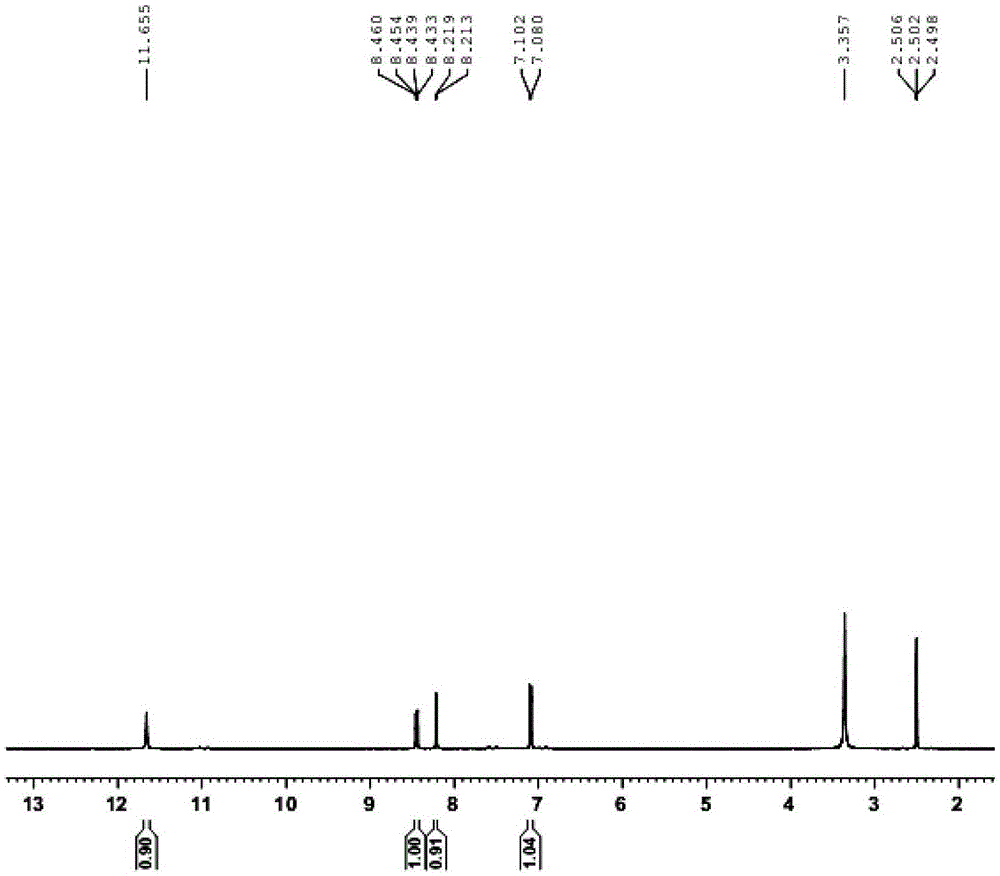
[0052] R 1 is methyl, R 2 For the synthesis of oxime group, i.e. 1-methyl-3-oxime-5-acetylaminoindolinone, the specific steps are as follows:
[0053] (1) Synthetic intermediate 5-nitroindoledione
[0054] Weigh 5.00g (33.98mmol) of isatin and 3.44g (33.98mmol) of potassium nitrate, dissolve them slowly in 20mL and 15mL of concentrated sulfuric acid respectively under stirring, and then dissolve isatin at 0~-4℃ Slowly drop the concentrated sulfuric acid solution into the concentrated sulfuric acid solution of potassium nitrate, continue to stir for 30 minutes after the dropwise addition, then stir at room temperature for 10 minutes, TLC detects that the reaction is complete, pour it into the ice-water mixture, stir for 30 minutes, and suction filter to obtain a yellow solid. After drying in a vacuum oven, 5.72 g of the target product 5-nitroindoledione was obtained with a yield of 87.6%.
[0055] 1 HNMR(DMSO400MHz):δ / ppm7.09(d,1H,J=8.8,ArH),8.21(s,1H,ArH),8.44(d,1H,J=8.8,A...
Embodiment 2
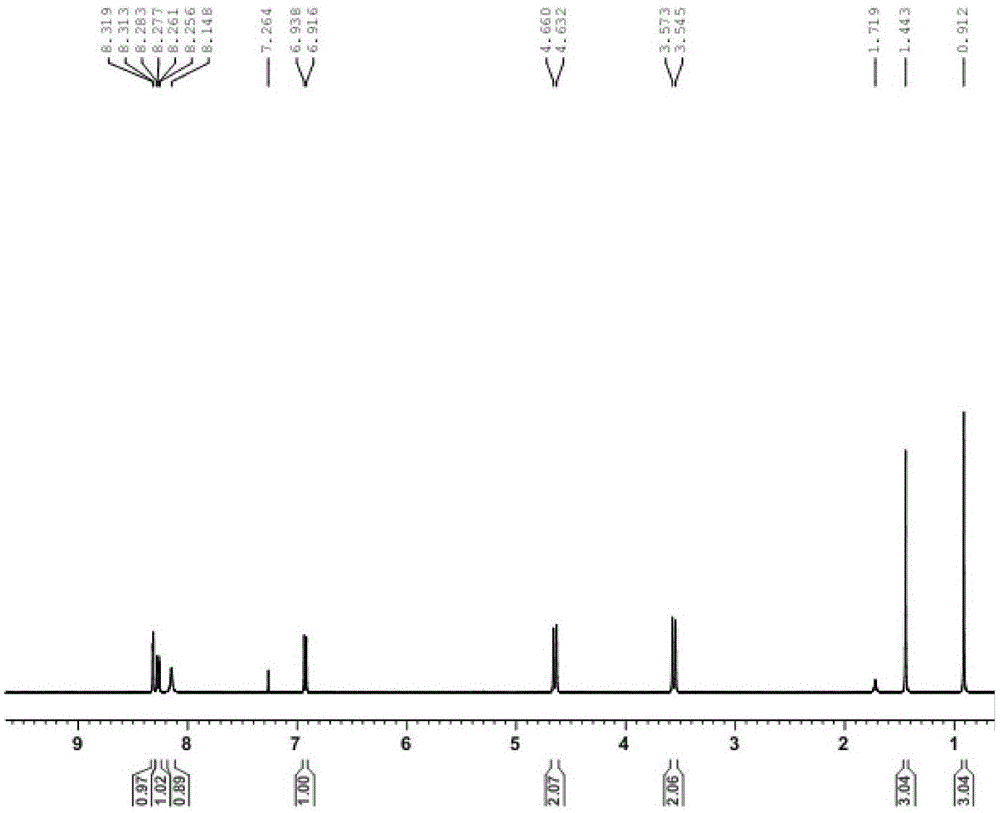
[0075] R 1 is 4-bromobenzyl, R 2 For the synthesis of oxime group, i.e. 1-(4-bromobenzyl)-3-oxime-5-acetamidolindolinone, the specific steps are as follows:
[0076] (1) Synthesis of intermediate 1-(4-bromobenzyl)-5-acetylaminoindoledione
[0077] Weigh 0.20 g (0.98 mmol) of 5-acetylaminoindolinone into a 25 mL round-bottomed flask, add 3 mL of N,N-dimethylformamide, and then slowly add 0.41 g of anhydrous potassium carbonate while stirring in an ice bath (2.94mmol), and finally added 0.27g (1.08mmol) of 4-bromobenzyl bromide, stirred at room temperature for 5min, and heated to reflux at 65°C for 2h. TLC detected that the reaction was complete, quenched by adding 15 mL of water, extracted three times with ethyl acetate, combined the organic phases, dried the organic phases with anhydrous sodium sulfate, and spun off the solvent under reduced pressure, petroleum ether: ethyl acetate = 2:1, 200 mesh Silica gel column chromatography purification. 0.31 g of 1-(4-bromobenzyl)-5...
Embodiment 3
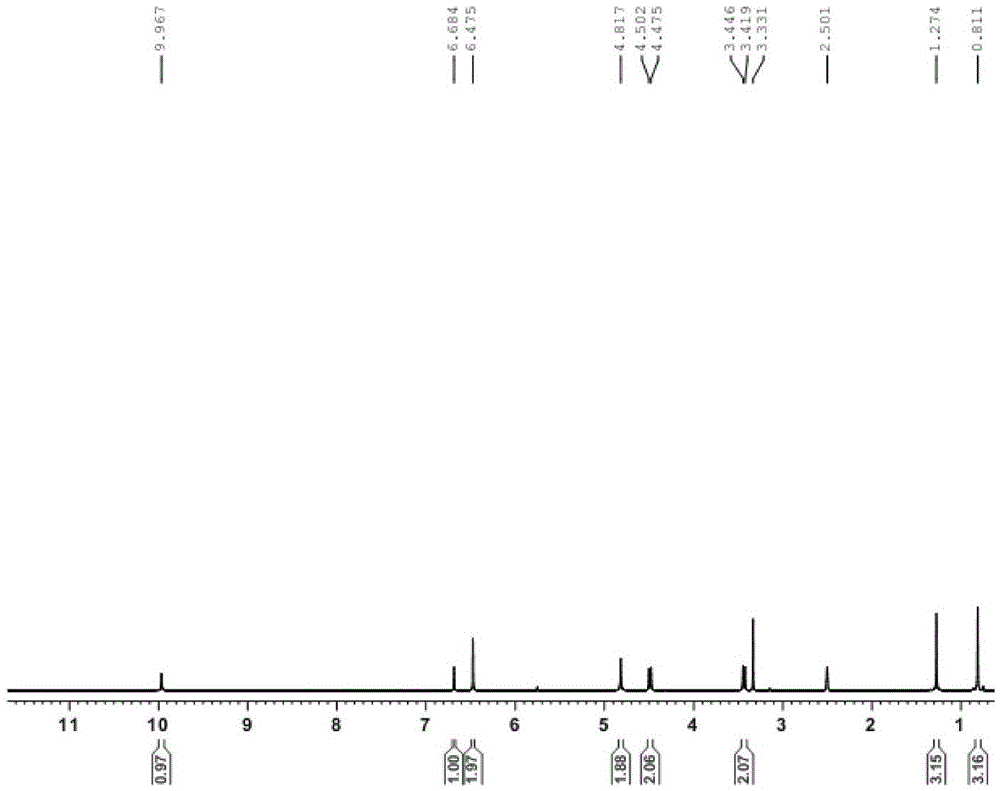
[0083] R 1 is 4-methylbenzyl, R 2For the synthesis of oxime group, i.e. 1-(4-methylbenzyl)-3-oxime-5-acetamidolindolinone, the specific steps are as follows:
[0084] (1) Synthesis of intermediate 1-(4-methylbenzyl)-5-acetylaminoindolinone
[0085] Weigh 0.20g (0.98mmol) of 5-acetylaminoindolinone into a 25mL round bottom flask, add 3mL N,N-dimethylformamide, and then slowly add 0.41g of anhydrous potassium carbonate while stirring in an ice bath (2.94mmol), and finally added 0.15g (1.08mmol) of 4-methylbenzyl chloride, stirred at room temperature for 5min, and heated to reflux at 65°C for 2h. TLC detected that the reaction was complete, quenched by adding 15 mL of water, extracted three times with ethyl acetate, combined the organic phases, dried the organic phases with anhydrous sodium sulfate, and spun off the solvent under reduced pressure, petroleum ether: ethyl acetate = 2:1, 200 mesh Silica gel column chromatography purification. 0.26 g of 1-(4-methylbenzyl)-5-acety...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com