MUC1 (Mucins) and IL-2 double-gene coexpression recombinant vector as well as preparation method and application thereof
A recombinant vector, IL-2 technology, used in gene therapy, recombinant DNA technology, and the introduction of foreign genetic material using vectors, can solve the problems of immune regulation, low anti-tumor ability, lack of immunotherapy targeting, etc. Enhance the expansion ability and cell viability, improve the effect of tumor immunotherapy, and expand the effect of anti-tumor immune effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1: Obtaining MUC1 gene fragments containing specific enzyme cleavage sites
[0050] 1. Primer design
[0051] According to the nucleotide sequence of the MUC1 gene (as shown in SEQ ID NO:1 in the sequence listing) and the expected insertion multiple cloning site on the pIRES2-EGFP plasmid vector, design specific primers as follows:
[0052] MUC1 upstream primer (as shown in SEQ ID NO:4 in the sequence listing):
[0053] 5'-GA AGATCT ATGACACCGGGCACCC-3' (the underlined part is the sequence of the Bgl II restriction site),
[0054] MUC1 downstream primer (as shown in SEQ ID NO:5 in the sequence listing):
[0055] 5'-TT GAATTC CTACAAGTTGGCAGAAGTGG-3' (the underlined part is the sequence of EcoR I restriction site).
[0056] 2. Obtain cDNA template
[0057] RNA was extracted from human breast cancer cell MCF-7 by TRIzon method (TRIzon total RNA extraction kit was purchased from Beijing Kangwei Century Biotechnology Co., Ltd., product number is CW0580), and r...
Embodiment 2
[0064] Example 2: Construction of pIRES2-MUC1-EGFP recombinant vector
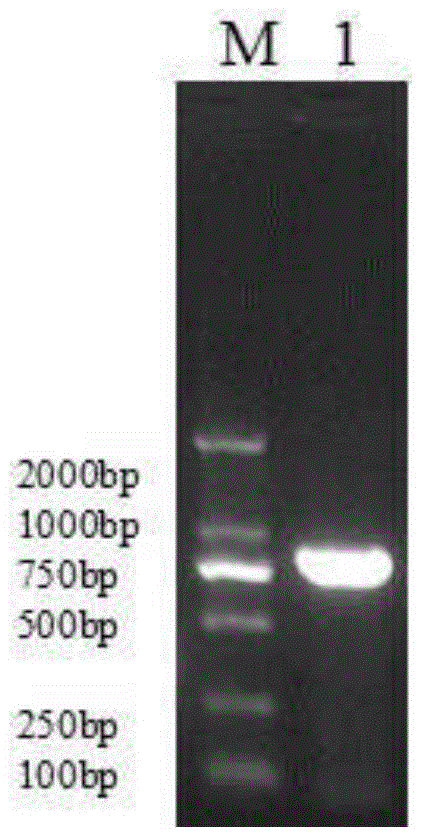
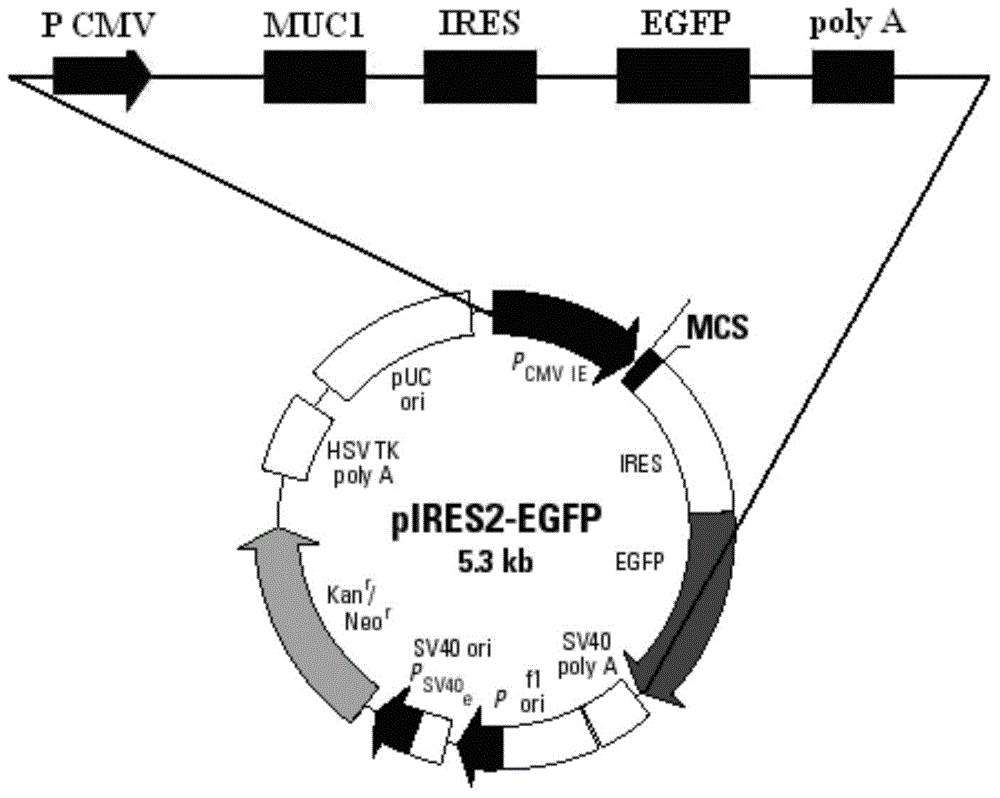
[0065] Using restriction endonucleases Bgl II and EcoR I, digest the pIRES2-EGFP plasmid (the multiple cloning site of the plasmid contains Bgl II, EcoR I restriction sites) and the MUC1 gene fragment obtained in Example 1, respectively, to obtain Linearized pIRES2-EGFP vector after digestion and MUC1 gene sequence after digestion; use T4 DNA ligase system for ligation reaction, incubate at 22°C for 30 minutes, and then inactivate at 70°C for 5 minutes to construct pIRES2-MUC1 -EGFP recombinant vector (such as image 3 shown).
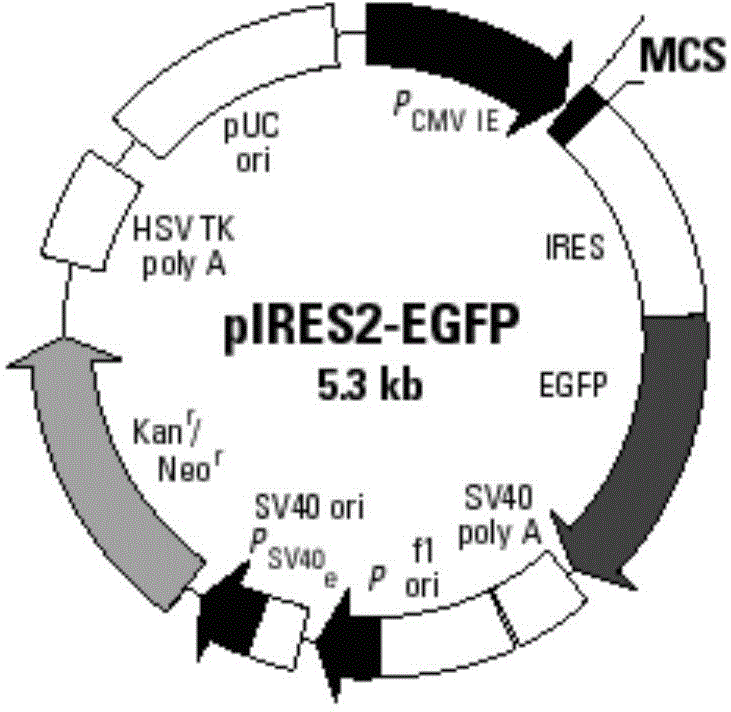
[0066] Structural features of the pIRES2-EGFP plasmid (eg figure 2 Shown) It can be seen that after the MUC1 gene is inserted into the multiple cloning site of the pIRES2-EGFP plasmid, it is located upstream of the self-sequence IRES of the plasmid vector (such as image 3 shown), that is, MUC1 and EGFP sequences were expressed separately under the same promoter.
[0067] 1. D...
Embodiment 3
[0093] Example 3: Double PCR method to obtain IL-2 gene fragment with restriction endonuclease sticky ends
[0094] According to the IL-2 gene sequence and the expected multi-cloning site on the pIRES2-EGFP plasmid vector, two pairs of primers with different lengths and sticky ends of restriction endonucleases were designed; reverse transcribed with RNA extracted from CIK cells The cDNA was used as a template, and the above-mentioned two pairs of primers were used for PCR amplification to obtain two PCR amplification products; after mixing the two PCR amplification products, denaturation and annealing were performed in turn to obtain four IL-2 gene fragments, wherein Both ends of the two IL-2 gene fragments have restriction endonuclease sticky ends, so that the IL-2 gene fragments can be directionally ligated to the intended polycloning of the plasmid vector without restriction endonuclease digestion in the site.
[0095] Compared with the traditional PCR product cloning meth...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com