Active substance for nonaqueous electrolyte secondary cell, method for producing active substance, electrode for nonaqueous electrolyte secondary cell, and nonaqueous electrolyte secondary cell
A non-aqueous electrolyte and secondary battery technology, applied in the direction of non-aqueous electrolyte batteries, battery electrodes, lithium batteries, etc., can solve the problems of insufficient initial efficiency, poor high-rate discharge performance, and no disclosure, etc., and achieve excellent initial efficiency , Large discharge capacity, excellent high rate discharge performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] The active material for a non-aqueous electrolyte secondary battery in Example 1 is produced by mixing the coprecipitated precursor that has been pre-baked as described above with a Li compound and then baking it. As the Li compound, it can be suitably produced by using lithium hydroxide, lithium carbonate, lithium nitrate, lithium acetate, or the like.
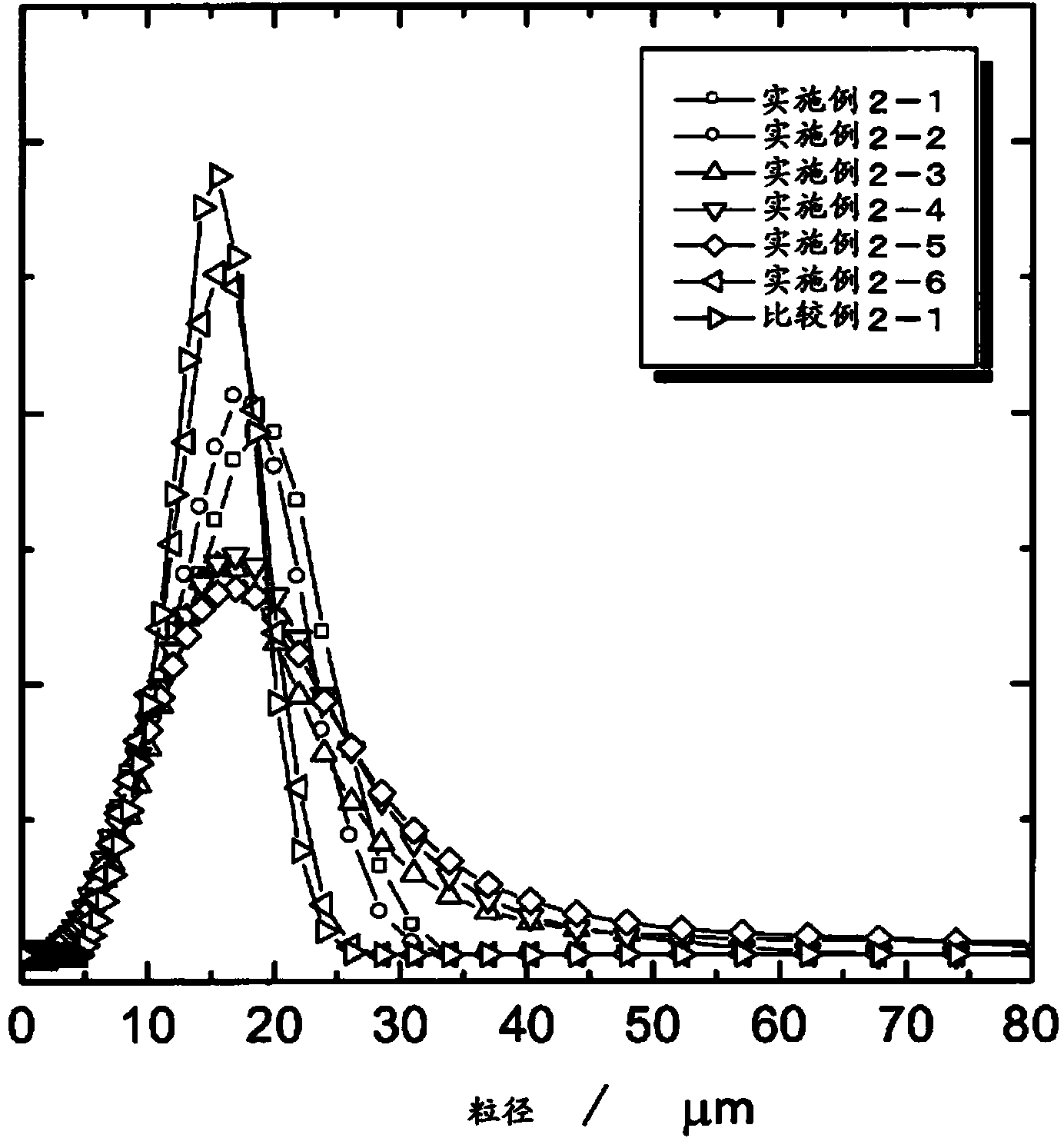
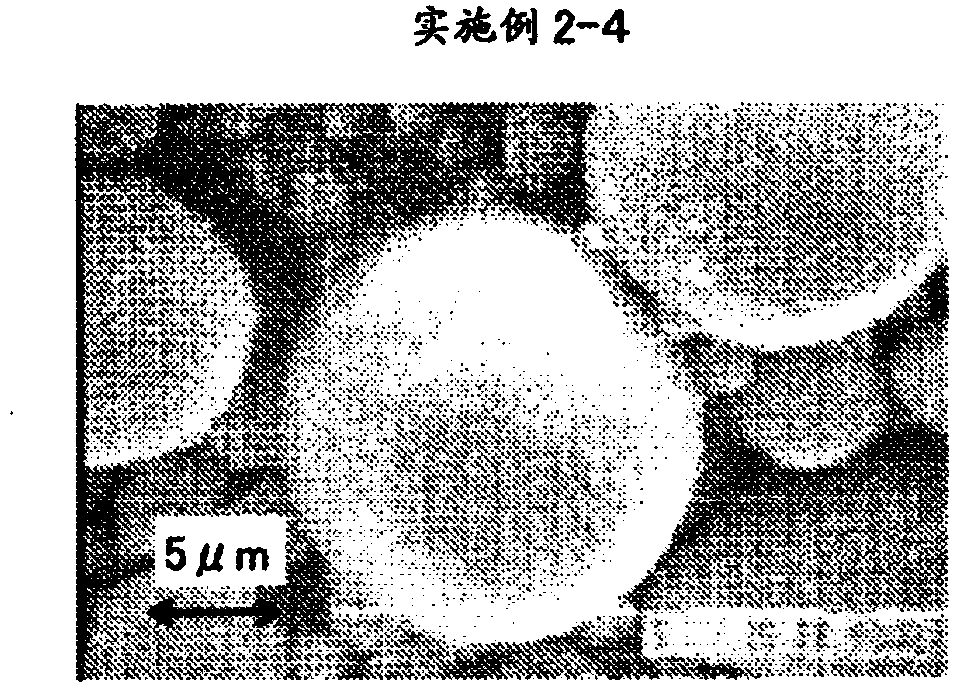
[0077] In Example 2, after coprecipitating a compound of the transition metal element Me containing Co, Ni, and Mn in a solution to produce a coprecipitated precursor of transition metal carbonate, the coprecipitated precursor and the lithium compound were mixed to prepare The powder is mixed, and the mixed powder is temporarily fired. As the lithium compound, lithium hydroxide, lithium carbonate, lithium nitrate, lithium acetate, etc. can be used, but lithium carbonate is preferred. The temperature of the temporary firing is preferably 250 to 750°C.
[0078] By performing temporary firing, the transition metal carbonate ...
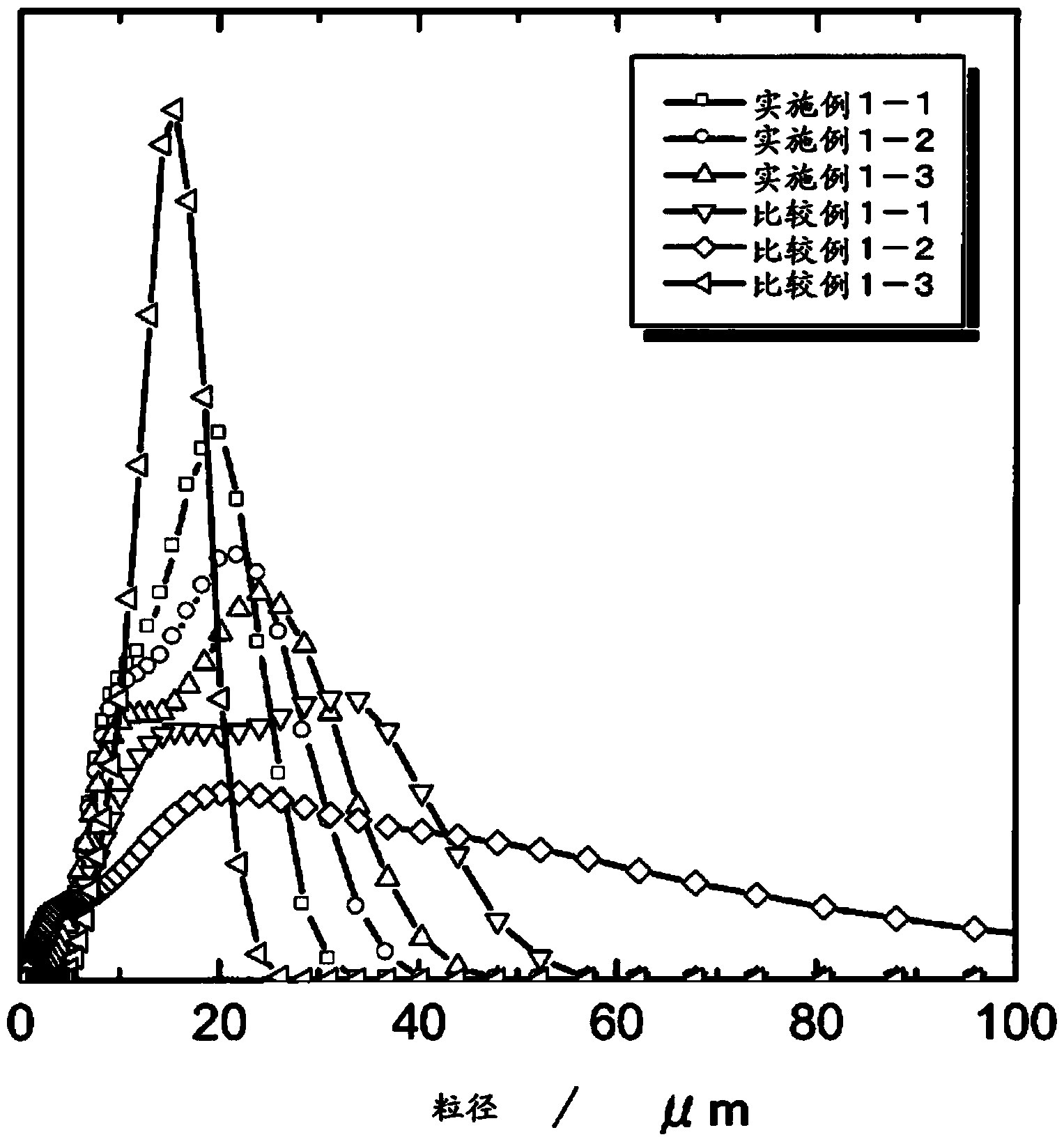
Embodiment 1-1)
[0109] 〔Synthesis of active substances〕
[0110] Weigh cobalt sulfate heptahydrate, nickel sulfate hexahydrate, and manganese sulfate pentahydrate with the molar ratio of Co, Ni and Mn of 12.5:19.94:67.56, and dissolve them in ion-exchanged water to produce 2M sulfate Aqueous solution. On the other hand, a 15L reaction tank was prepared. The reaction tank is provided with a discharge port so that when the liquid level inside the reaction tank exceeds a certain height, the solution is discharged from the discharge port. In addition, a stirring blade is provided in the reaction tank, and a cylindrical convection plate for generating vertical convection during stirring is fixed. Add 7L of ion exchange water to the above reaction tank to make CO 2 The gas is bubbled for 30 minutes, so that the above CO 2 The gas is fully dissolved in ion exchange water. It should be stated that CO 2 The gas bubbling continued until the end of the dripping of the aqueous sulfate solution. Next, th...
Embodiment 1-2)
[0115] Except that the temporary firing temperature of the co-precipitated carbonate precursor was changed to 400°C, the lithium transition metal composite oxide according to Example 1-2 was produced in the same manner as Example 1-1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap