Electrode active material for lithium secondary battery and method for manufacturing same
A technology of electrode active materials and lithium secondary batteries, which is applied in secondary battery manufacturing, battery electrodes, non-aqueous electrolyte battery electrodes, etc., can solve the problems of initial efficiency degradation and low initial efficiency, and achieve improved conductivity and good rate performance, effect of minimizing dead volume
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069]
[0070] 50 parts by weight of lithium chloride (LiCl) was dissolved in ethanol, and 50 parts by weight of SiO were uniformly dispersed therein to obtain a dispersion liquid. The dispersion liquid was heated to 70° C. to remove the solvent used, thereby obtaining a mixture. The mixture was annealed at 800° C. under Ar atmosphere to prepare the nucleating material according to the present invention.
[0071]
[0072] 40mmol resorcinol, 40mmol formaldehyde (3000ml37% by weight aqueous solution), 0.75mmol sodium carbonate as catalyst and 9.88mmol cetyltrimethylammonium bromide (CTAB) as dispersant are dispersed in 1000ml distilled water, 10 g of a nucleating material was added thereto, followed by heating at 85° C. for 3 days, thereby obtaining a resorcinol-formaldehyde (RF) sol solution.
[0073] The RF sol containing the nucleation material obtained above was annealed at 900° C. for 5 hours under an Ar atmosphere, thereby forming a conductive carbon layer on the sur...
Embodiment 2
[0080] The procedure of Example 1 was repeated except that the coating of the conductive carbon material was performed by using methane as a raw material in a rotary tube furnace, thereby preparing an electrode active material and a battery.
[0081] The formation of the conductive carbon coating using methane is specifically carried out as follows.
[0082] 20 g of nucleating material was introduced into a rotary tube furnace where argon was supplied at a rate of 0.5 L / min and the temperature of the furnace was raised up to 900 °C at a rate of 5 °C / min. While supplying 1.8 L / min of argon gas and 0.3 L / min of methane gas, heat treatment was performed by rotating a rotary tube furnace at 10 rpm for 5 hours, thereby preparing an electrode active material having a conductive carbon coating.
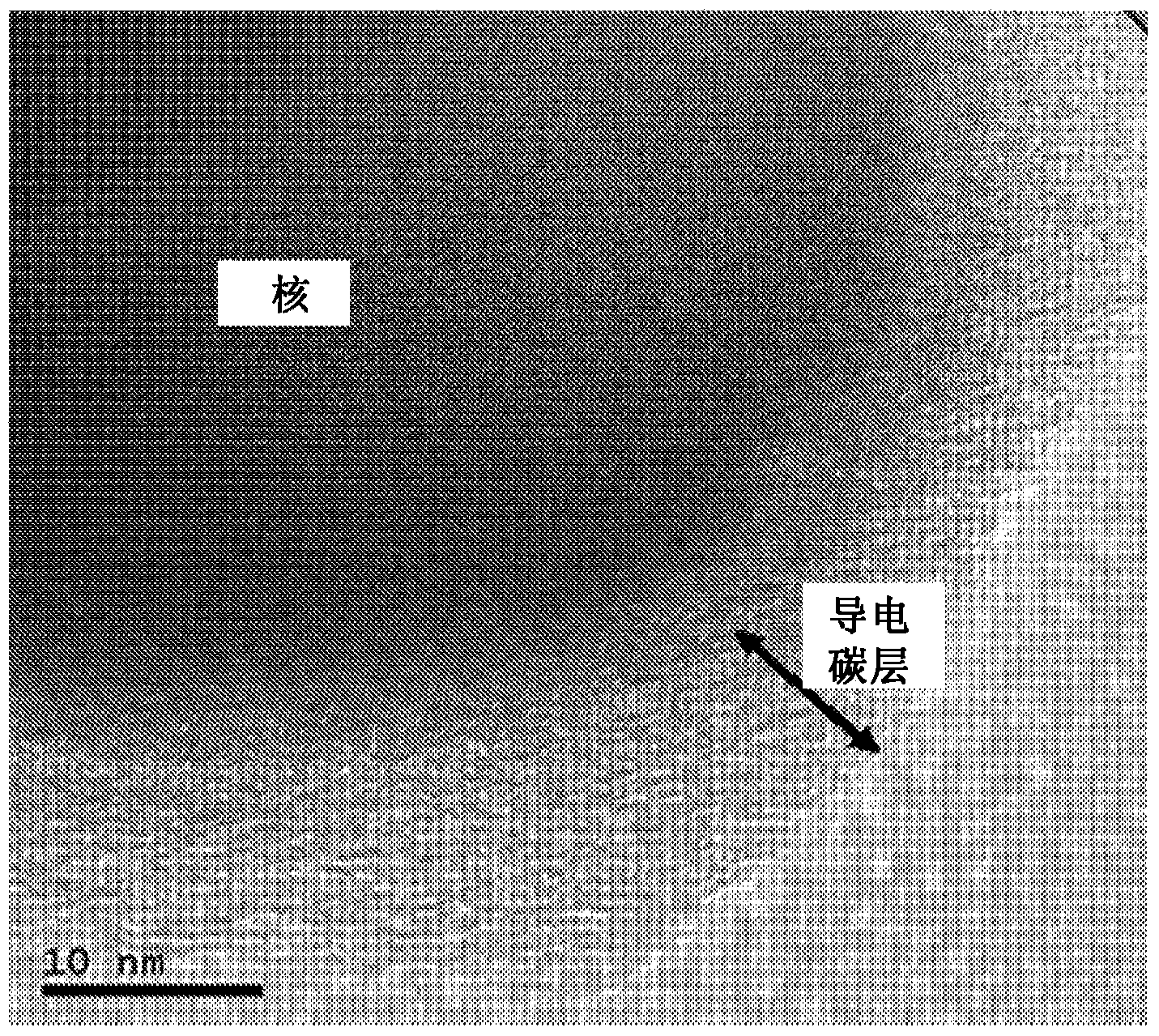
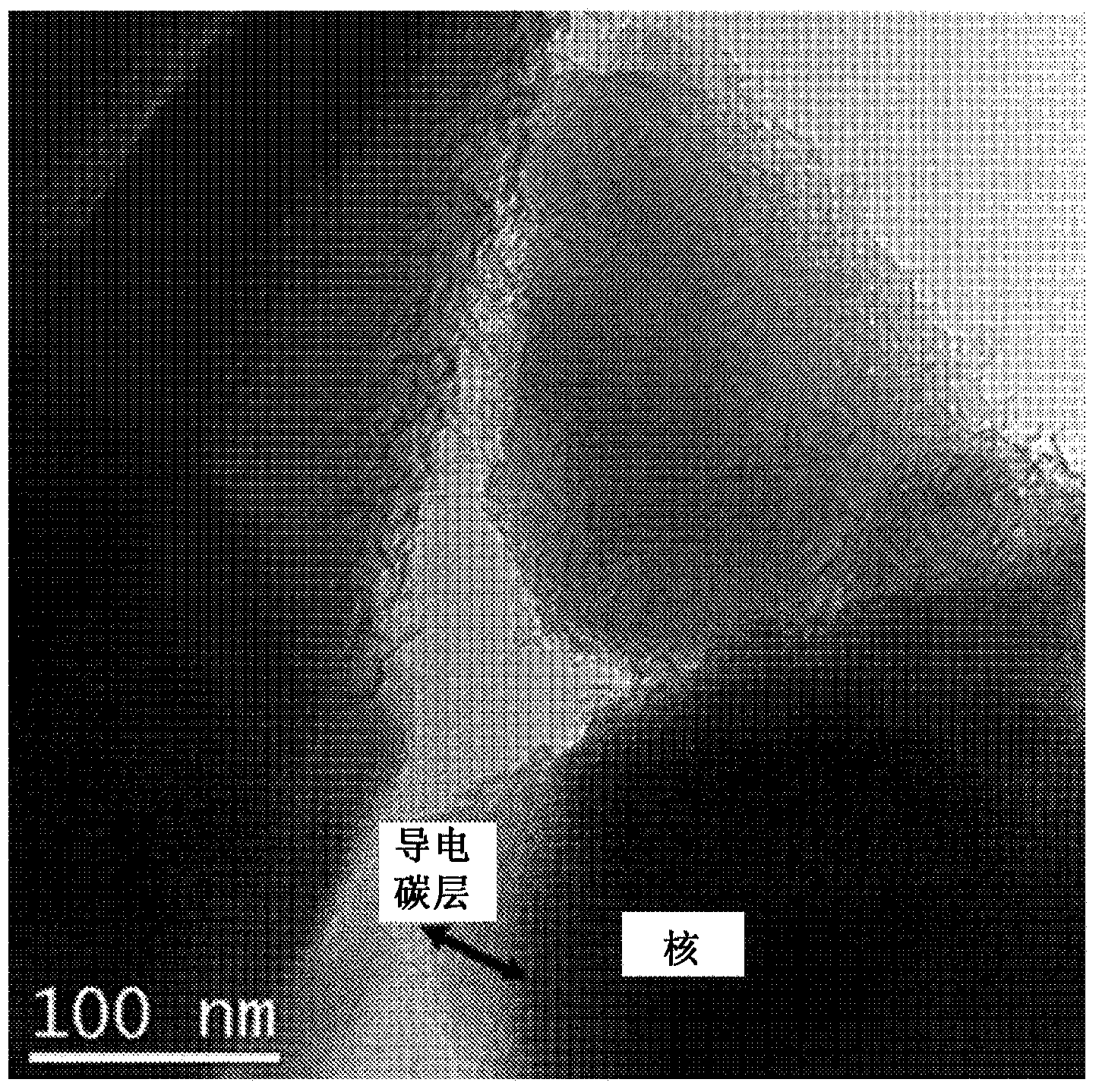
[0083] It was confirmed that the amount of conductive carbon in the conductive carbon layer was 5 parts by weight based on 100 parts by weight of the nucleating material. The TEM photo of t...
Embodiment 3
[0085] The procedure of Example 1 was repeated except that the coating of the conductive carbon material was carried out by mixing 100 parts by weight of the nucleating material with 10 parts by weight of D 50 = 15 μm of artificial graphite was mixed; with a 5:1 weight ratio, stainless steel balls with a diameter of 3 mm and the powder of the resulting mixture were put into a mechanical fusion device (Hosokawa Micron); then at 600 rpm Mechanical alloying was carried out and continued for 30 minutes.
[0086] The TEM photo of the electrode active material with conductive carbon layer according to the present invention is shown in image 3 middle.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap