Assays for influenza virus hemagglutinins
A technology for influenza virus and samples, which is applied in the experimental field of influenza virus hemagglutinin, such as analyzing vaccines, can solve the problem of uncertain detection of inactivated vaccine antigens
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
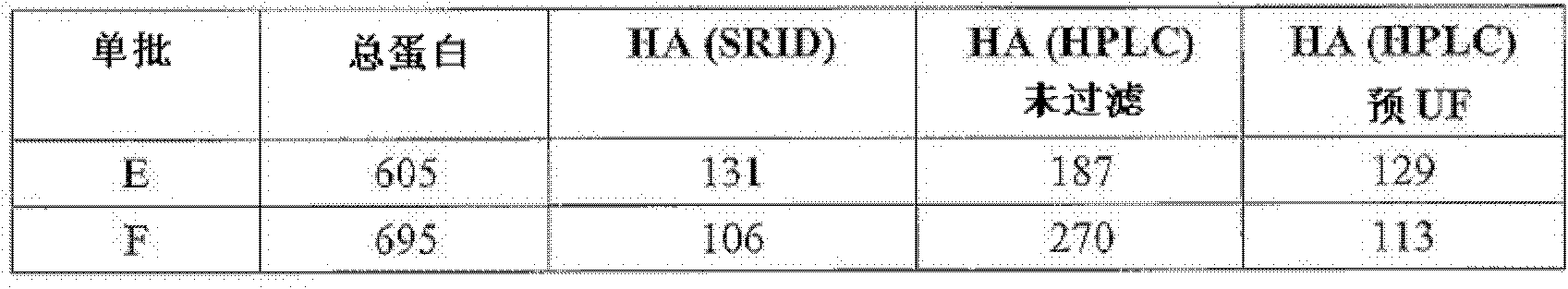
[0106] RP-HPLC detection of influenza HA as a method for the quantification of batches ("single batches") of monovalent influenza virus antigens. It was found that when the single batch had high specific purity and stable HA, RP-HPLC was able to quantify HA well, and the quantitative results closely matched the standard SRID results. But in cases where the vaccine contained significant amounts of denatured HA, the RP-HPLC method no longer matched the SRID assay.
[0107] For example, the table below shows the results of four A / H3N2 single batches. Total protein concentration ([mu]g / ml) was assessed by BCA, followed by HA concentration ([mu]g / ml) by SRID (standard protocol) and RP-HPLC. RP-HPLC on 2.1mm x 100mm Poros TM Performed on a R1 / 10 column operating at 60°C and a flow rate of 0.8ml / min. The mobile phases were: (A) 0.1% TFA in water, 5% acetonitrile; and (B) 0.1% TFA in 100% acetonitrile (solvent B), the A / B mixture was changed from 20% in 6.5 minutes / 80% becomes 0...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap