Method of forming an aromatic polyamide copolymer
A technology of polymers and oligomers, which is applied in the field of preparing aromatic polyamide polymers, and can solve problems such as the control of the position of monomer components that do not have
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
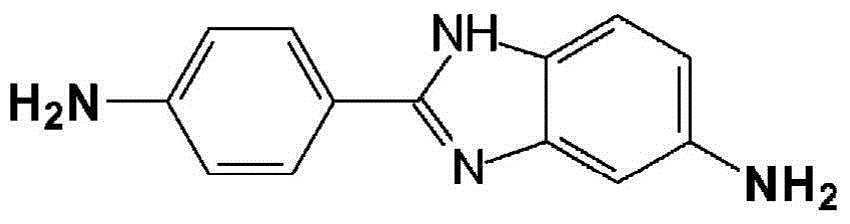
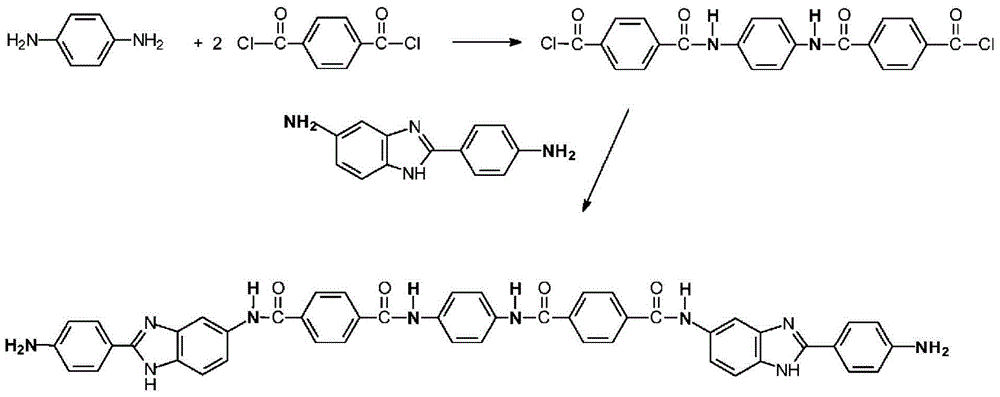
[0062] NMP, DMAC, LiCl, CaCl 2 , DAPBI, PPD and TCl were obtained from commercial sources.
example 1
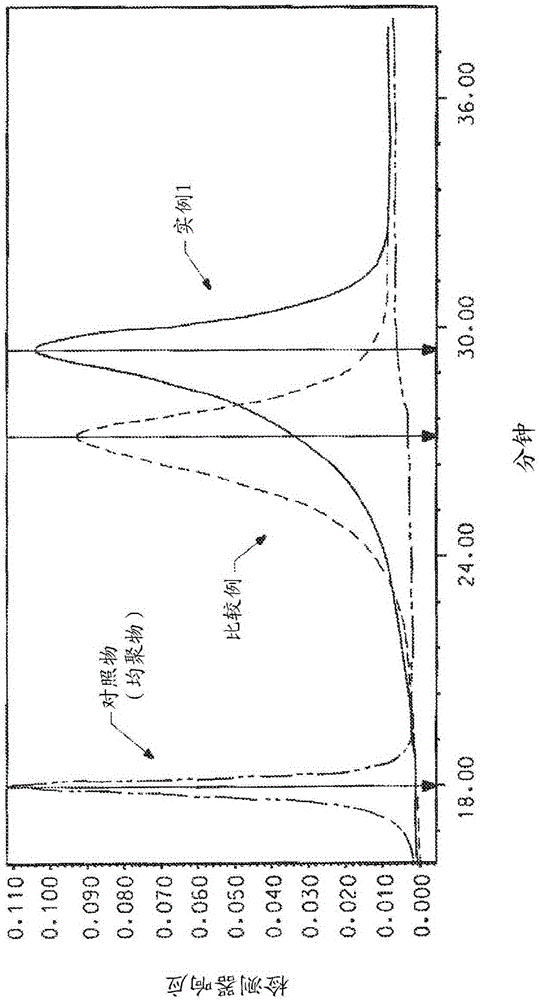
[0064] Add 104.64 grams of NMP / CaCl to a 1-liter reactor equipped with a frame stirrer and nitrogen inlet / outlet 2 Premix (8.3% by weight (weight of salt / total weight of salt and solvent)), 177.09 grams of NMP (N-methyl-2-pyrrolidone), and 2.539 grams (0.023 mole) of PPD (p-phenylenediamine), and Stir for 10 minutes. The contents were stirred in an ice water bath to cool the mixture to below 10°C. 9.536 g (0.047 mol) of TCl was added all at once and stirred for 5 minutes. The ice water bath was removed and 12.288 g (0.055 mole) DAPBI was added and stirred. The solution became very viscous and gelled in about 4 minutes. The highly viscous reaction mixture was stirred for another 25 minutes. Transfer the resulting polymer to Blender and grind into small particles, and then wash several times to remove solvent (NMP / CaCl 2 ) And excess HCl produced by the reaction. Then the polymer was neutralized with sodium bicarbonate, and finally washed with water several times to obtain a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com