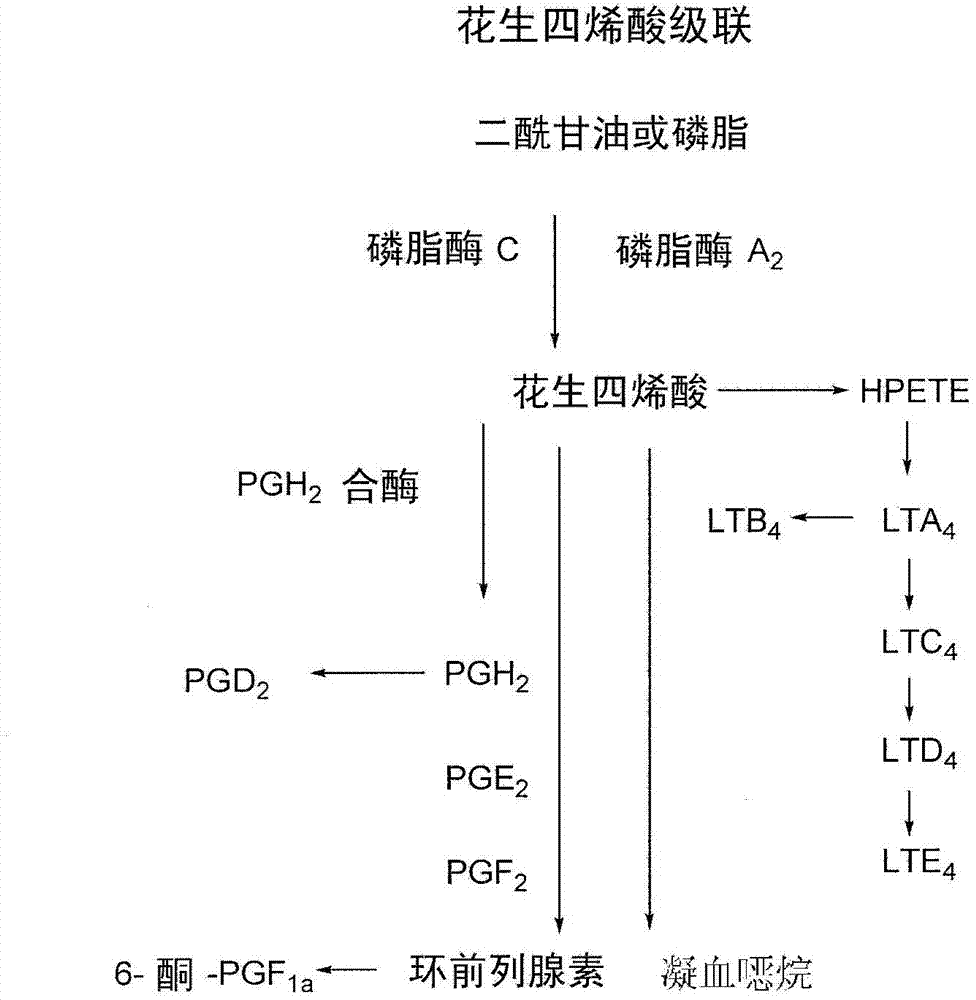
Treatment and prevention of diseases mediated by microorganisms via drug-mediated manipulation of the eicosanoid balance
A technology of microorganisms and antimicrobial agents, applied in the direction of resistance to vector-borne diseases, drug combinations, drug delivery, etc., can solve problems such as development, disease recurrence, drug resistance, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
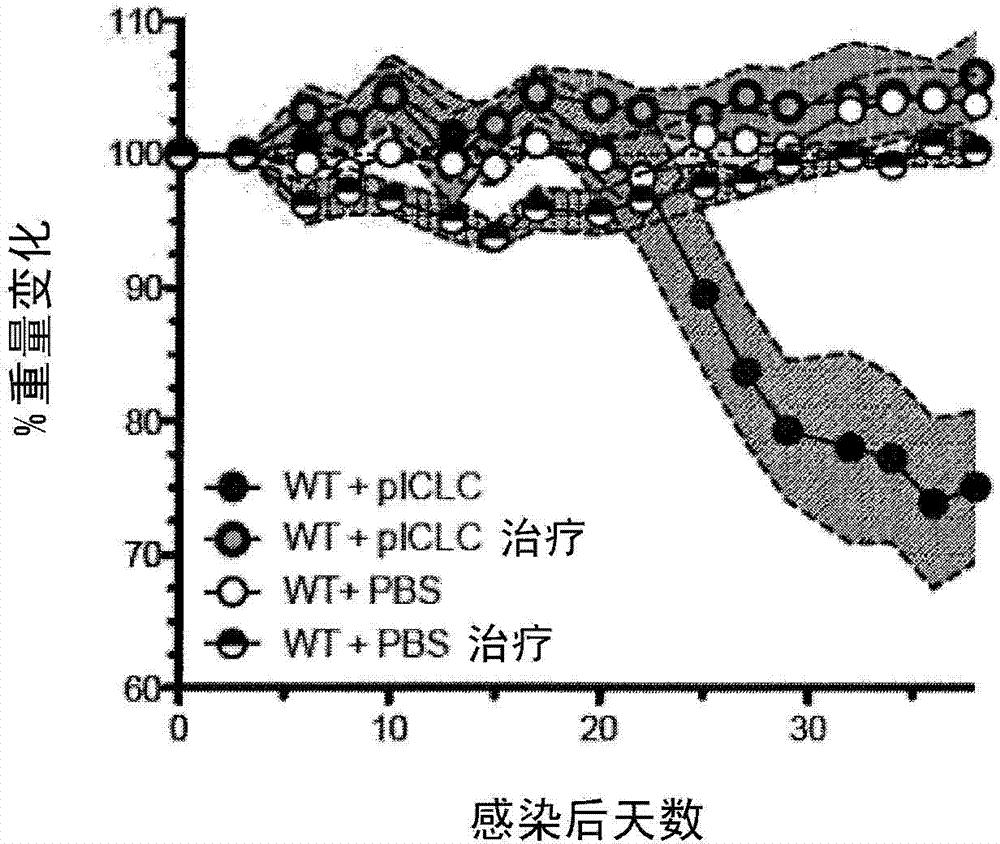
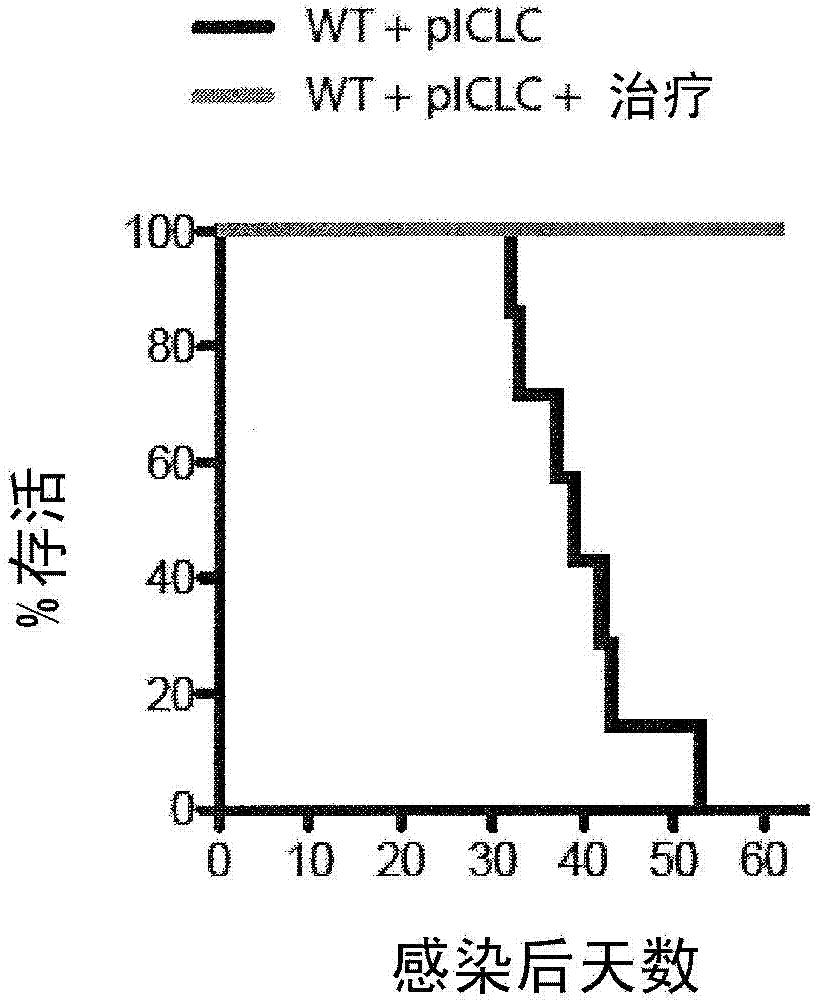
[0085] This example demonstrates the effect of co-administration of zileuton and PGE2 on C57BL6 mice infected with M. tuberculosis and simultaneously treated with poly-ICLC.
[0086] Four groups of five C57BL / 6 mice ("B6 mice") were used in this study. All four groups were exposed to M. tuberculosis at levels of 100-150 colony forming units via the intranasal aerosol route. A control group of five mice received no further treatment. The comparison group was treated twice weekly via intranasal administration of poly-ICLC, which is polyinosine-polycytidylic acid condensed with poly-L-lysine and carboxymethylcellulose (Oncovir Inc. , Washington, DC). A comparison group of five mice received no further treatment. Five mice in the test group were administered with zileuton (administered at a concentration of 6 mg / mL in drinking water), PGE2 (administered intranasally at a concentration of 6 μg / 30 μL in phosphate-buffered saline per mouse, twice a week) ) and poly-ICLC treatment...
Embodiment 2
[0092] Two groups of five IL-1a / bDKO- / - (IL-1α / β double knockout) mice and a group of five C57BL / 6 mice were used in this study. C57BL / 6 mice were used as controls.
[0093] All three groups were exposed to levels of 100-150 colony forming units of M. tuberculosis via the intranasal aerosol route. Five IL-1a / bDKO- / - mice in the test group and C57BL / 6 mice in the control group were treated with zileuton (administered at a concentration of 6 mg / mL in drinking water) and PGE2 (in phosphate A concentration of 6 μg / 30 μL in saline buffered saline administered intranasally twice a week) for treatment. The IL-1a / bDKO- / - mice in the comparison group were not treated with zileuton and PGE2.
[0094] At 40 days post-infection, no IL-1a / b DKO- / - mice of the comparison group survived. One of the five IL-la / bDKO- / - mice in the test group died on day 40, and the remaining four mice survived more than 40 days but less than about 65 days. All C57BL / 6 mice in the control group survived for...
Embodiment 3
[0098] C57BL6 were infected by aerosol route with 200 CFU of Mtb and poly-ICLC were administered twice weekly. As a control, a group of mice was treated with PBS and not poly-ICLC. The second group of mice received no further treatment. A third group of mice was further treated with PGE2. The fourth group of mice was further treated with PGE2 and zileuton. A fifth group of mice was further treated with zileuton alone.
[0099] After a period of time, the colony forming units ("CFU") in the lungs of each group of mice were determined and the results are graphically shown in Figure 5 middle.
[0100] as in Figure 5 From the results plotted in it is clear that the control group has about 7.4log 10 CFU. Mtb-infected poly-ICLC-treated mice had approximately 8.9 log 10 CFU. Mtb-infected poly-ICLC treated mice further treated with PGE2 had approximately 9.2 log 10 CFU. Mtb-infected poly-ICLC treated mice further treated with PGE2 had approximately 8.9 log 10 CFU. Mtb-i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com