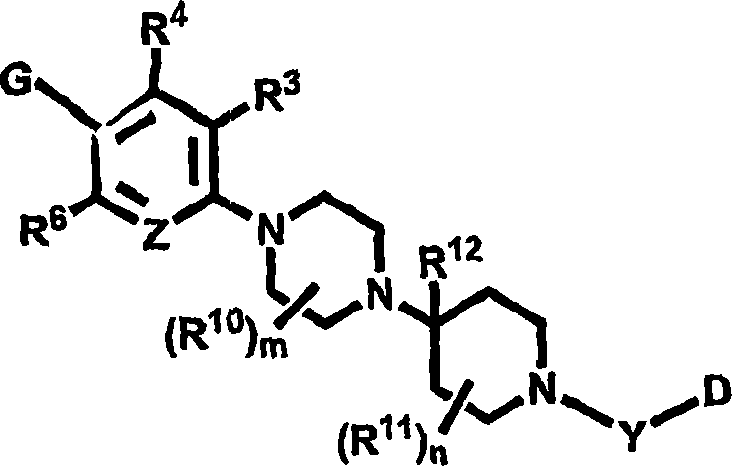
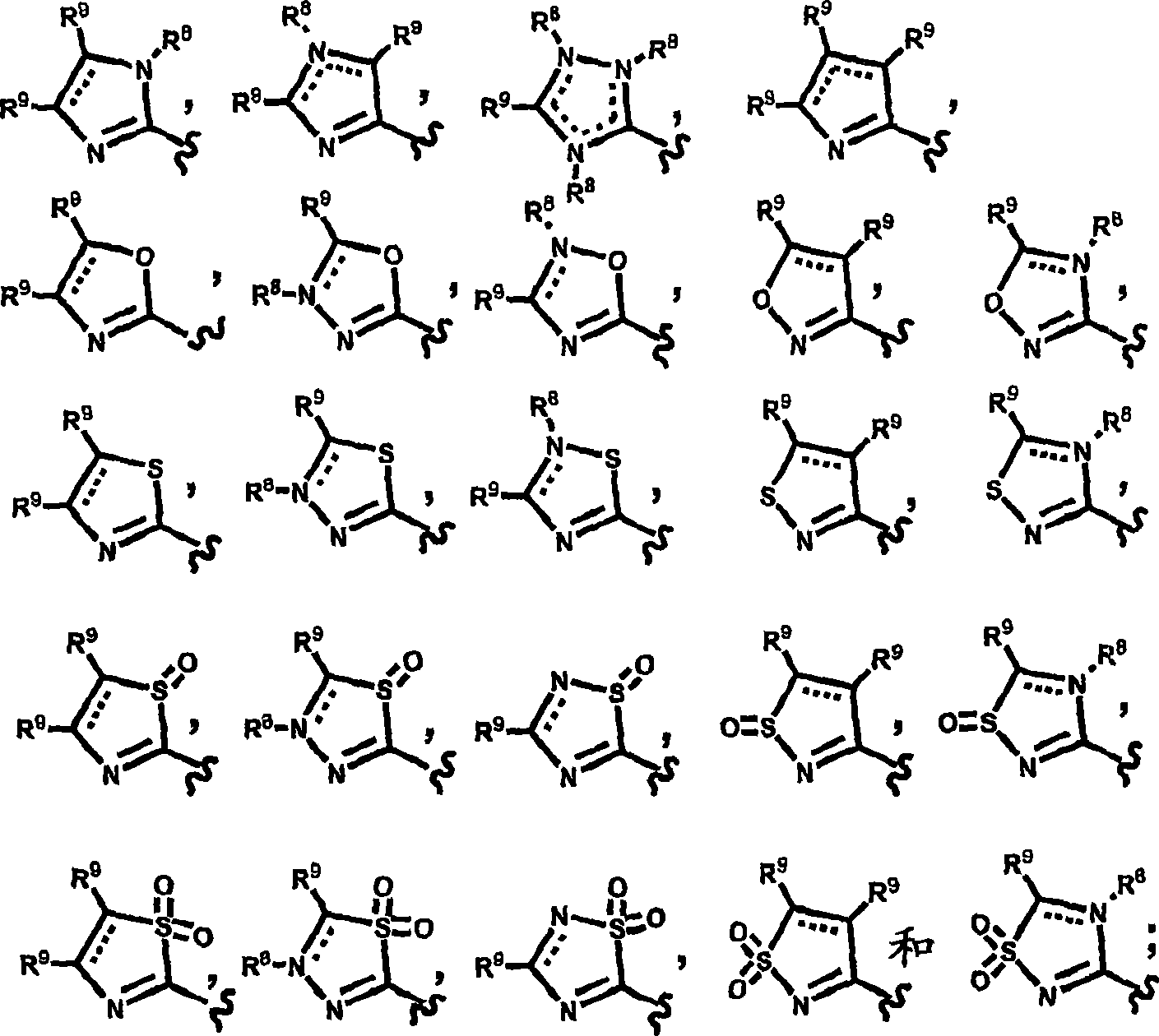
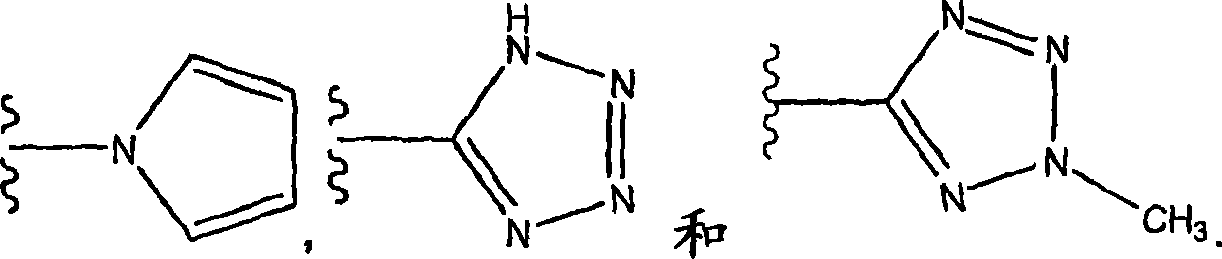
Novel heterocyclic substituted pyridine or phenyl compounds with CXCR3 antagonist activity
A compound, heterocyclic group technology, applied in the field of new heterocyclic substituted pyridine or phenyl compounds with CXCR3 antagonist activity, can solve the problems of not identifying the receptor, not showing the effect, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0461] Preparation Example 1. EN=CO 2 PR 1 =CO 2 CH 3
[0462]
[0463] 2,3-Dichloropyridine 5-carboxylate methyl ester (5,6-dichloronicotinic acid methyl ester, BIONET) was prepared from 5,6-dichloronicotinic acid (ALDRICH): by using excess SOCl 2 Work-up and reflux for 1.5 hours converted the acid to the acid halide followed by methylation in near quantitative yield in methanol / pyridine as solvent. Ref. Musso et al., Bioorg. Med. Chem. Lett., 1997, 7, 1-6.
[0464] The round bottom flask was loaded with methyl 2,3-dichloropyridine 5-carboxylate 1 (7 g, 34 mmol), 2-S-ethylpiperazine (as prepared by Williams et al. J. Med. Chem 1996, 39, 1345) (75% active, 5.2g, 34mmol), cesium carbonate (12.15g, 68mmol), DBPD (0.74g, 2.48mmol), palladium acetate (0.55g, 2.48mmol) and 1,4 dioxane (170ml). The flask was equipped with a reflux condenser and heated to 80 °C. After 36 hours, the reaction was cooled, diluted with dichloromethane (-200ml) and washed with water (2x50ml). T...
preparation Embodiment 2
[0465] Preparative Example 2. EN = Nitrile
[0466]
[0467] 2,3-Dichloro5-cyanopyridine 4 was prepared from 5,6-dichloronicotinic acid (ALDRICH). by using excess SOCl 2 Work-up and reflux for 1.5 hours converted the acid to the acid halide. Reaction of the intermediate acid chloride with ammonia (aq) affords the primary amide, which is treated with excess SOCl at reflux 2 Dehydration was carried out to obtain 2,3-dichloro-5-cyanopyridine 4. [Ref George et al. J.Org.Chem, 1979, 44, 2697.]
[0468]The round bottom flask was loaded with 2,3-dichloro5-cyanopyridine 4 (20 g, 95.5 mmol), 2-S-ethylpiperazine (as prepared by Williams et al. J. Med. Chem 1996, 39, 1345) (80% active, 13.7g, 95.5mmol), cesium carbonate (62.2g, 191mmol), DBPD (2.07g, 6.97mmol), palladium acetate (1.56g, 6.97mmol) and 1,4 dioxane (475ml). The flask was equipped with a reflux condenser and heated to 80°C under nitrogen. After 48 hours, the reaction was cooled, diluted with dichloromethane (-200ml) ...
preparation Embodiment 3
[0470] Preparative Example 3. EN = Nitrile
[0471]
[0472] The flask was loaded with 5 (16.3 g, 65.2 mmol), N-Boc piperidin-4-one (16.88 g, 84.76 mmol) and 1,2-dichloroethane (170 ml) and stirred at 60° C. for 20 minutes. Add reducing agent NaB(OAc) slowly under stirring 3 H (1.5 equiv). The resulting suspension was stirred at 60° C. for 3 days, then treated with saturated sodium bicarbonate solution to pH=13, extracted with dichloromethane and dried over magnesium sulfate. The solvent was removed under reduced pressure and the residue was purified by flash chromatography on silica gel using 1.5% then 5.0% methanol in dichloromethane as eluent to afford 7 (25.1 g, 89%). MS: m / e, M+H=434.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com