Patents
Literature
62 results about "CXCR3" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
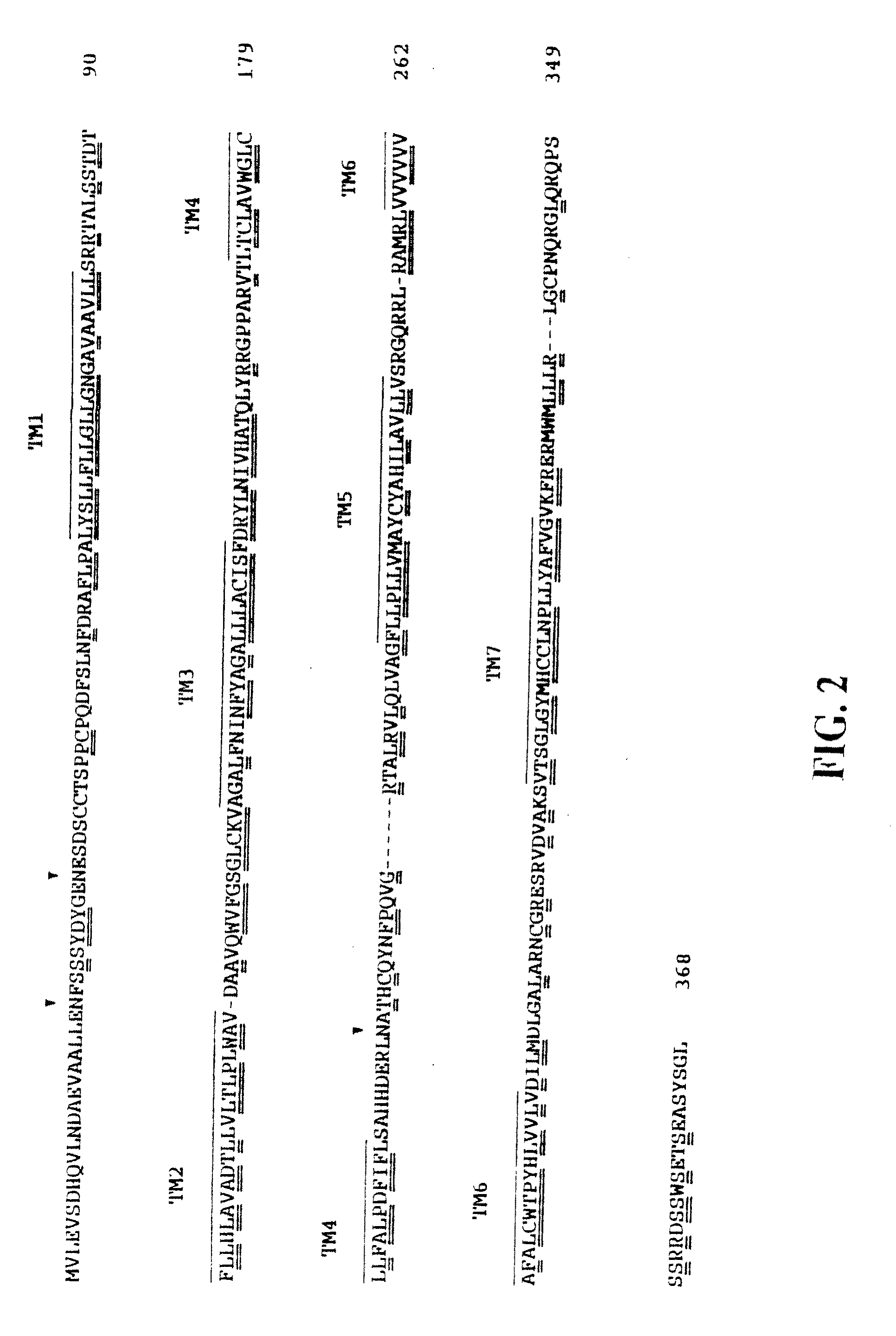
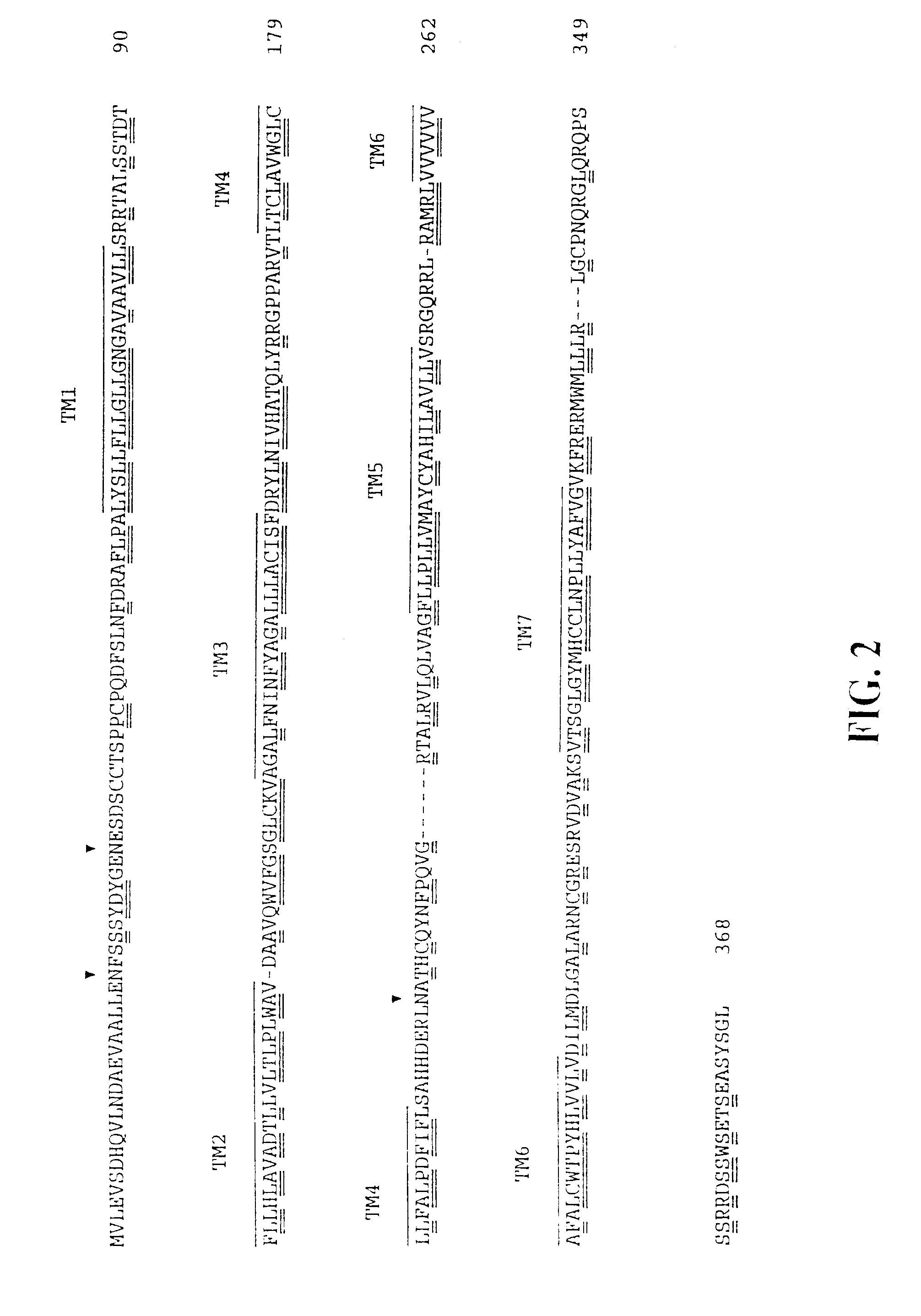
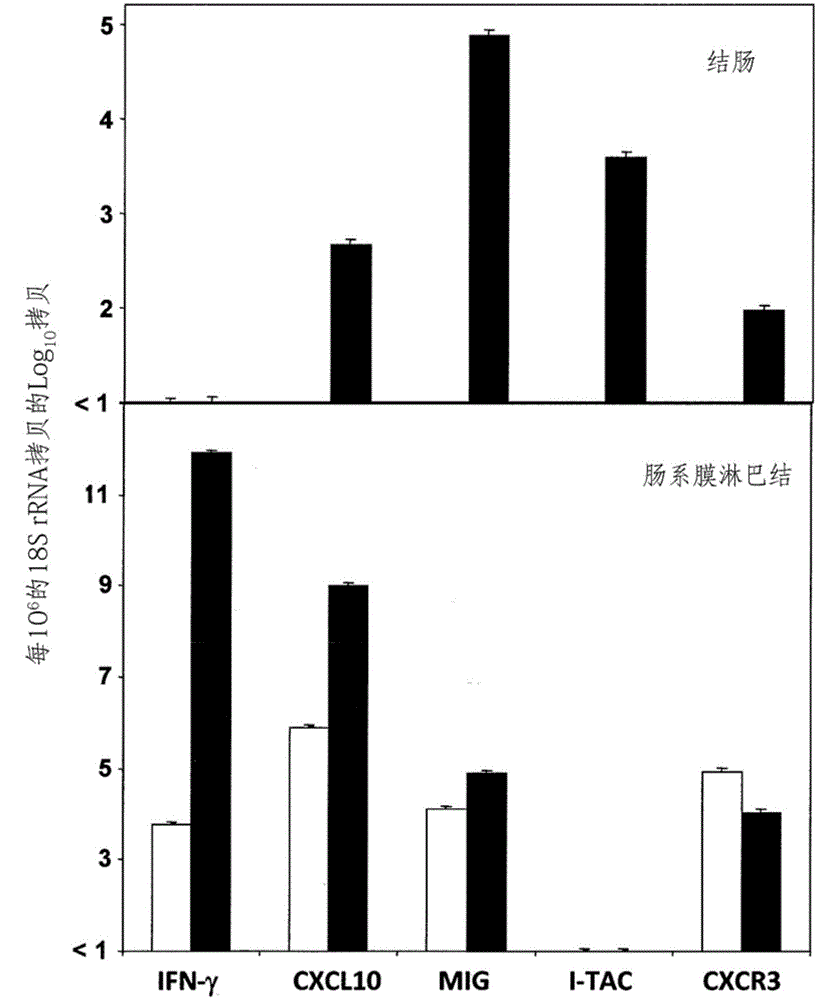
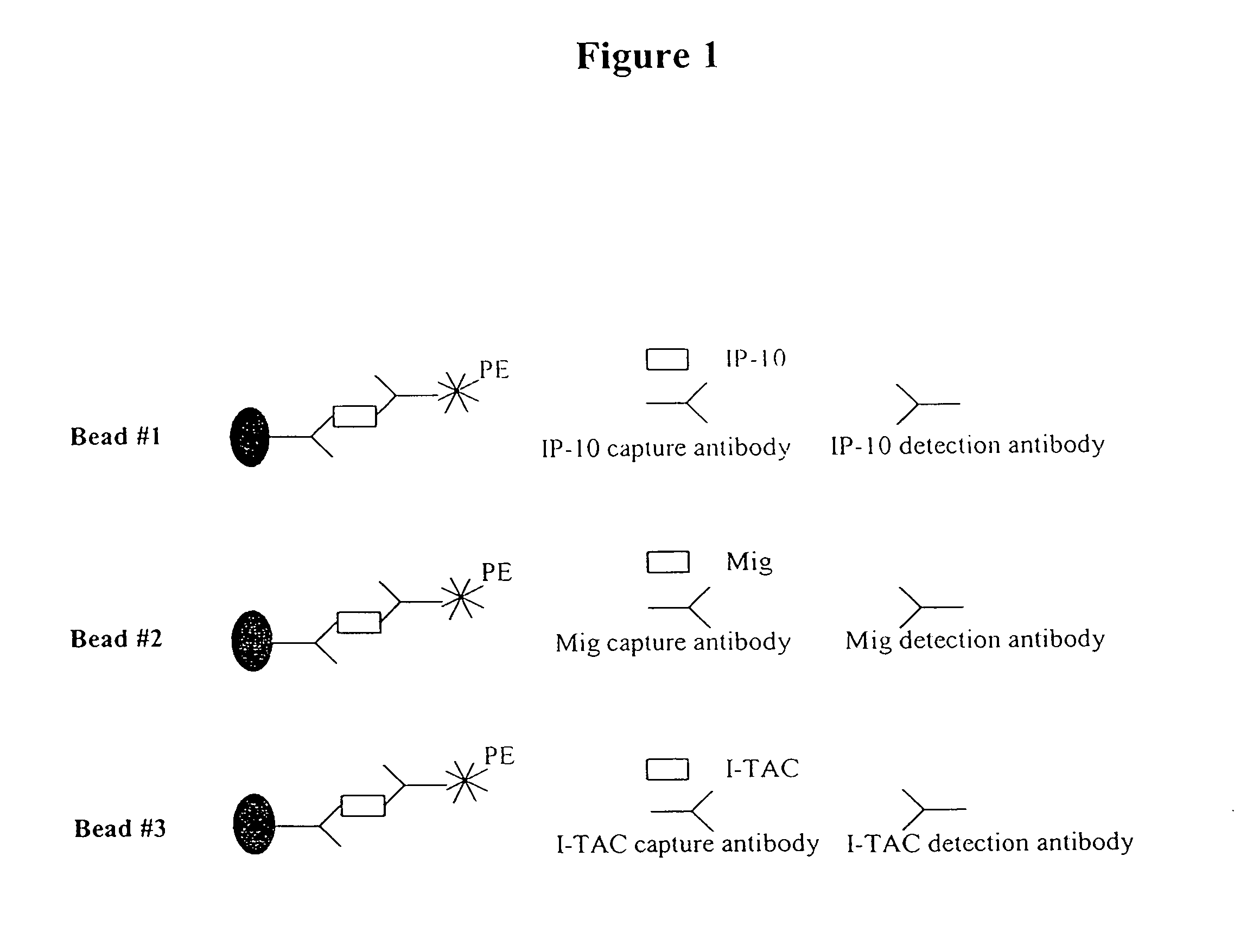
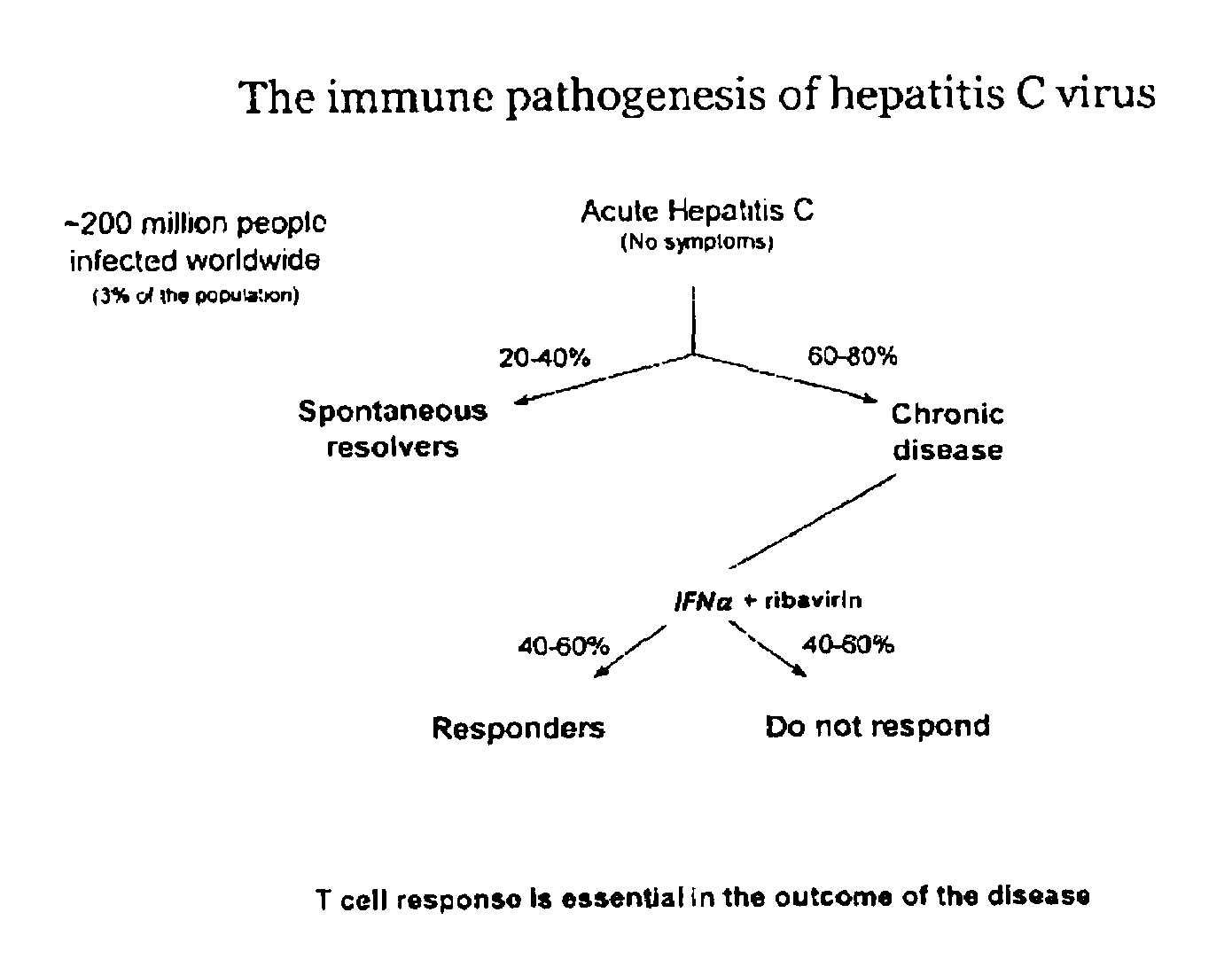
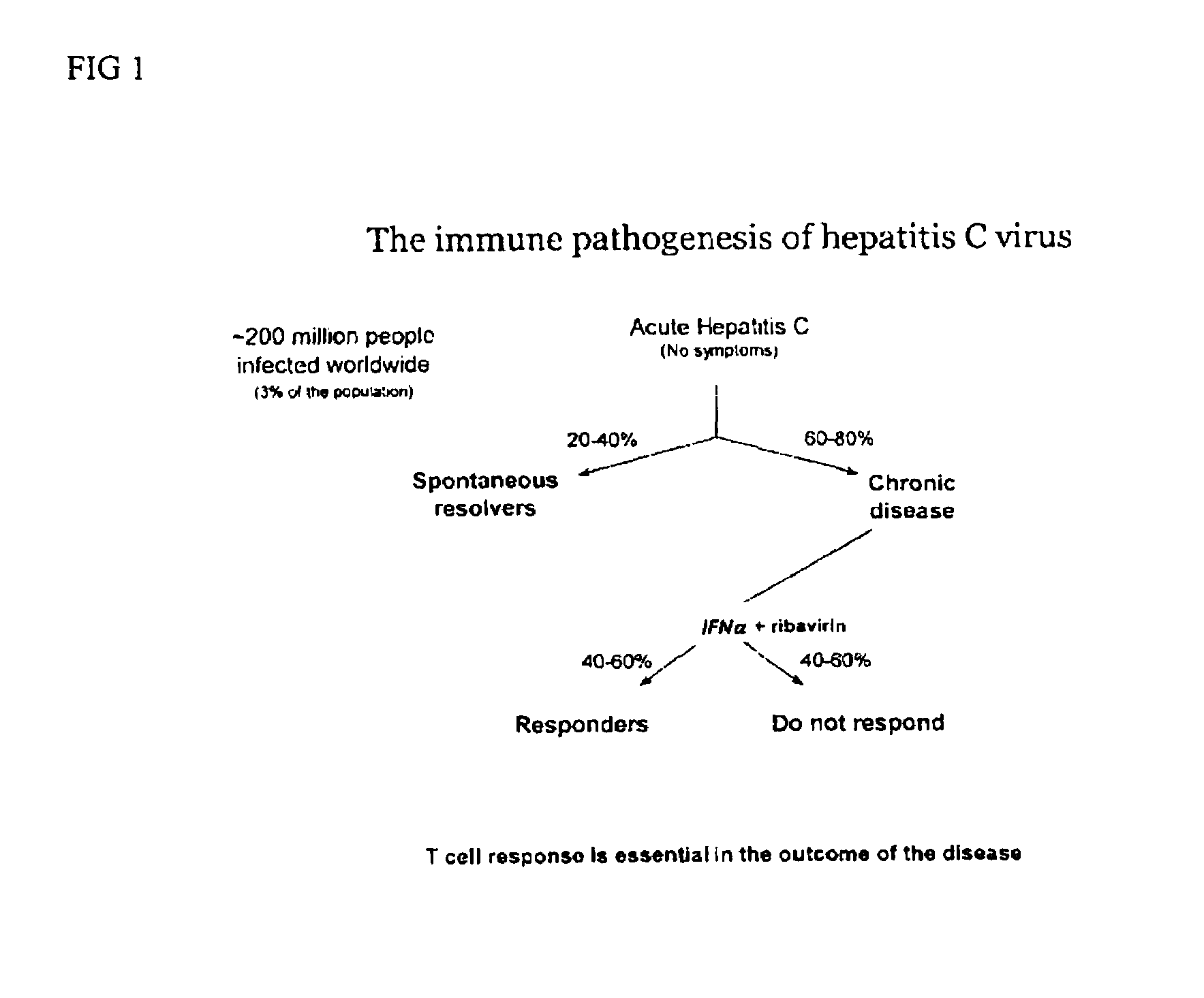
Chemokine receptor CXCR3 is a Gαᵢ protein-coupled receptor in the CXC chemokine receptor family. Other names for CXCR3 are G protein-coupled receptor 9 (GPR9) and CD183. There are three isoforms of CXCR3 in humans: CXCR3-A, CXCR3-B and chemokine receptor 3-alternative (CXCR3-alt). CXCR3-A binds to the CXC chemokines CXCL9 (MIG), CXCL10 (IP-10), and CXCL11 (I-TAC) whereas CXCR3-B can also bind to CXCL4 in addition to CXCL9, CXCL10, and CXCL11.
Method of treating graft rejection using inhibitors of CXCR3 function
InactiveUS20020018776A1Improve survivabilityPrevent graft rejectionBiocideBiological material analysisCo administrationSurgery
A method for inhibiting the rejection of transplanted grafts is disclosed. The method comprising administering an effective amount of an antagonist of CXCR3 function to a graft recipient. The disclosed methods can also comprise the co-administration of one or more additional therapeutic agents, for example, immunosuppressive agents.
Owner:MILLENNIUM PHARMA INC
Novel antagonists of cxcr3-binding cxc chemokines
InactiveUS20060204498A1Reduced tendency to interactAntibacterial agentsPeptide/protein ingredientsAutoimmune conditionAutoimmune disease
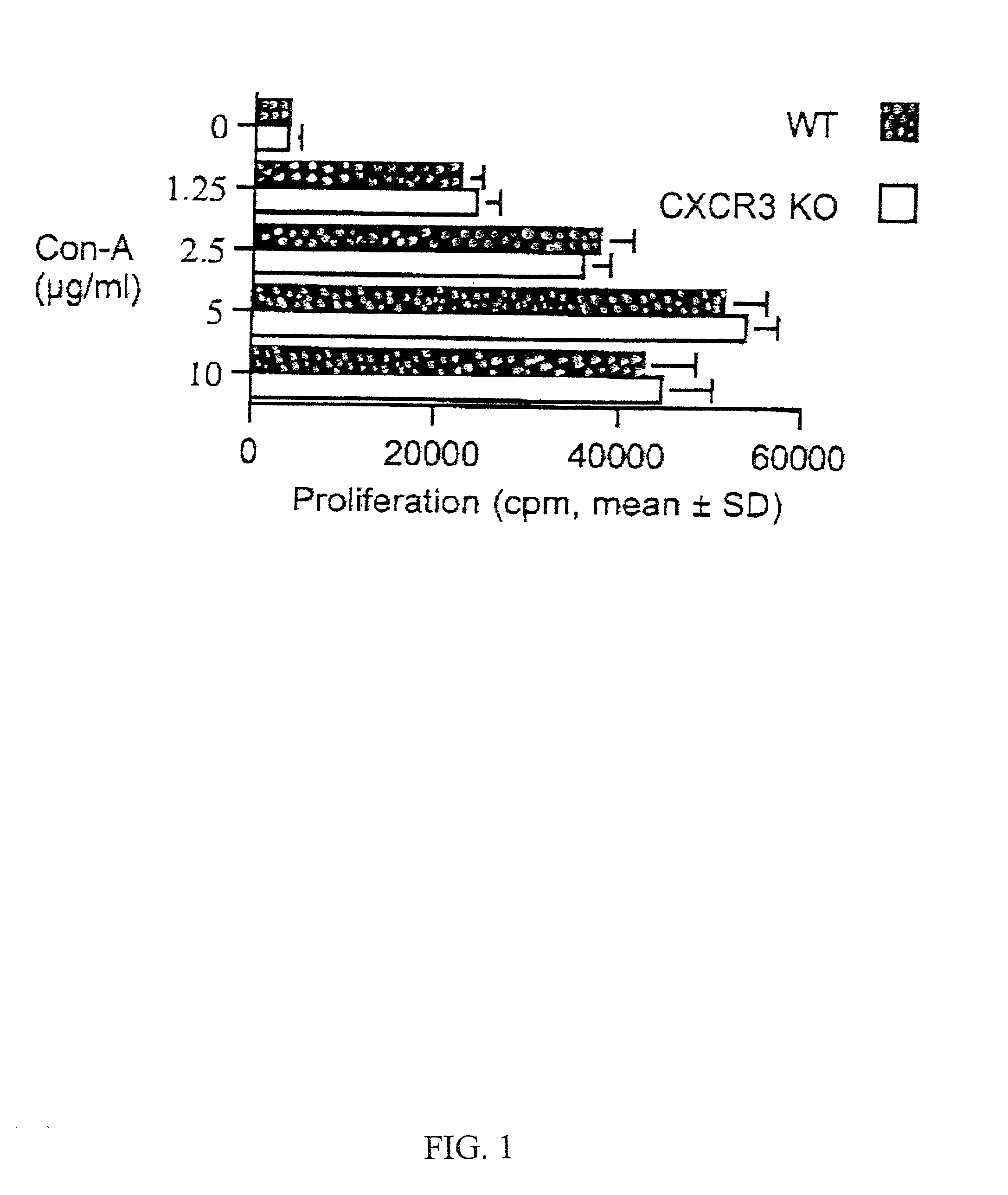
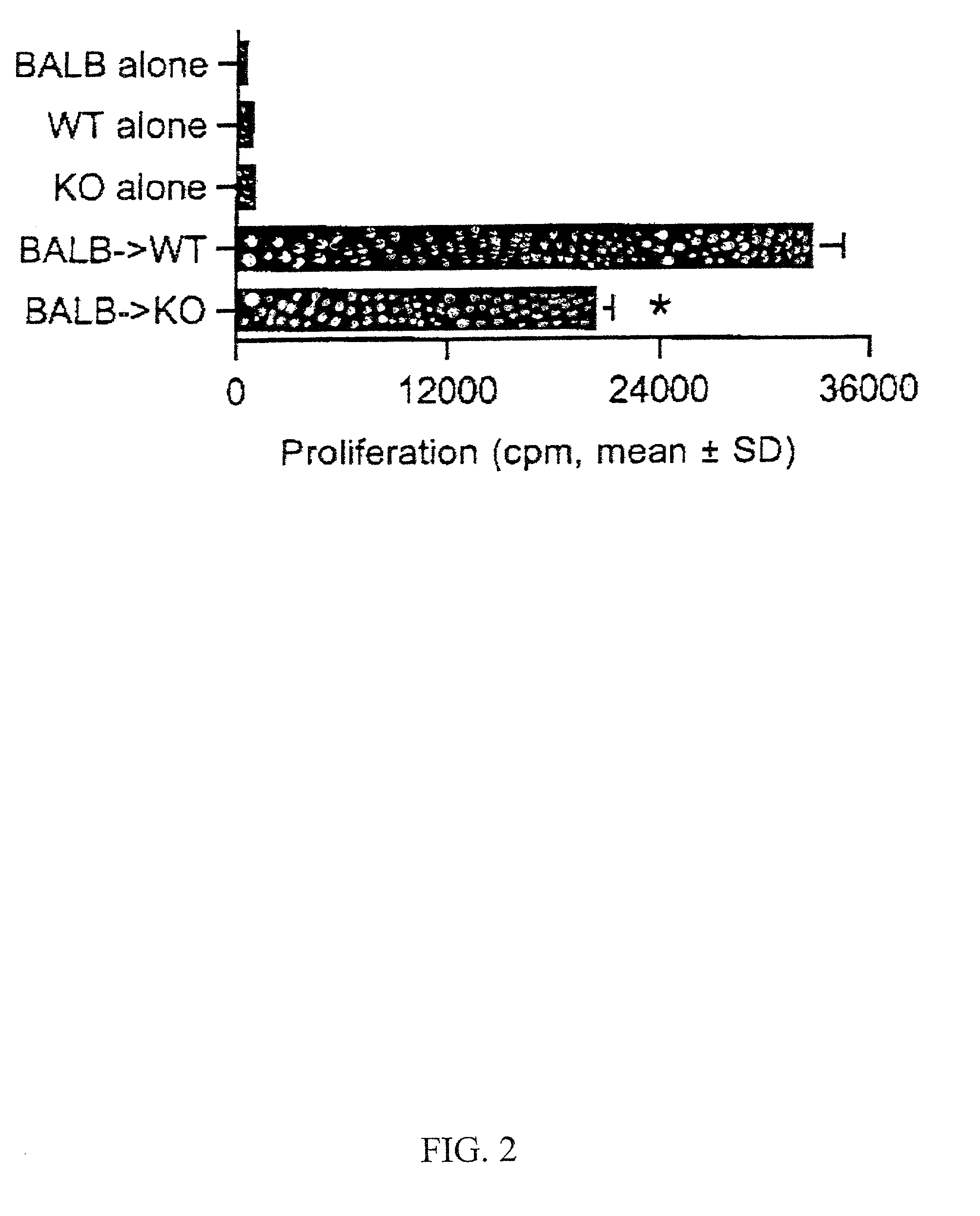
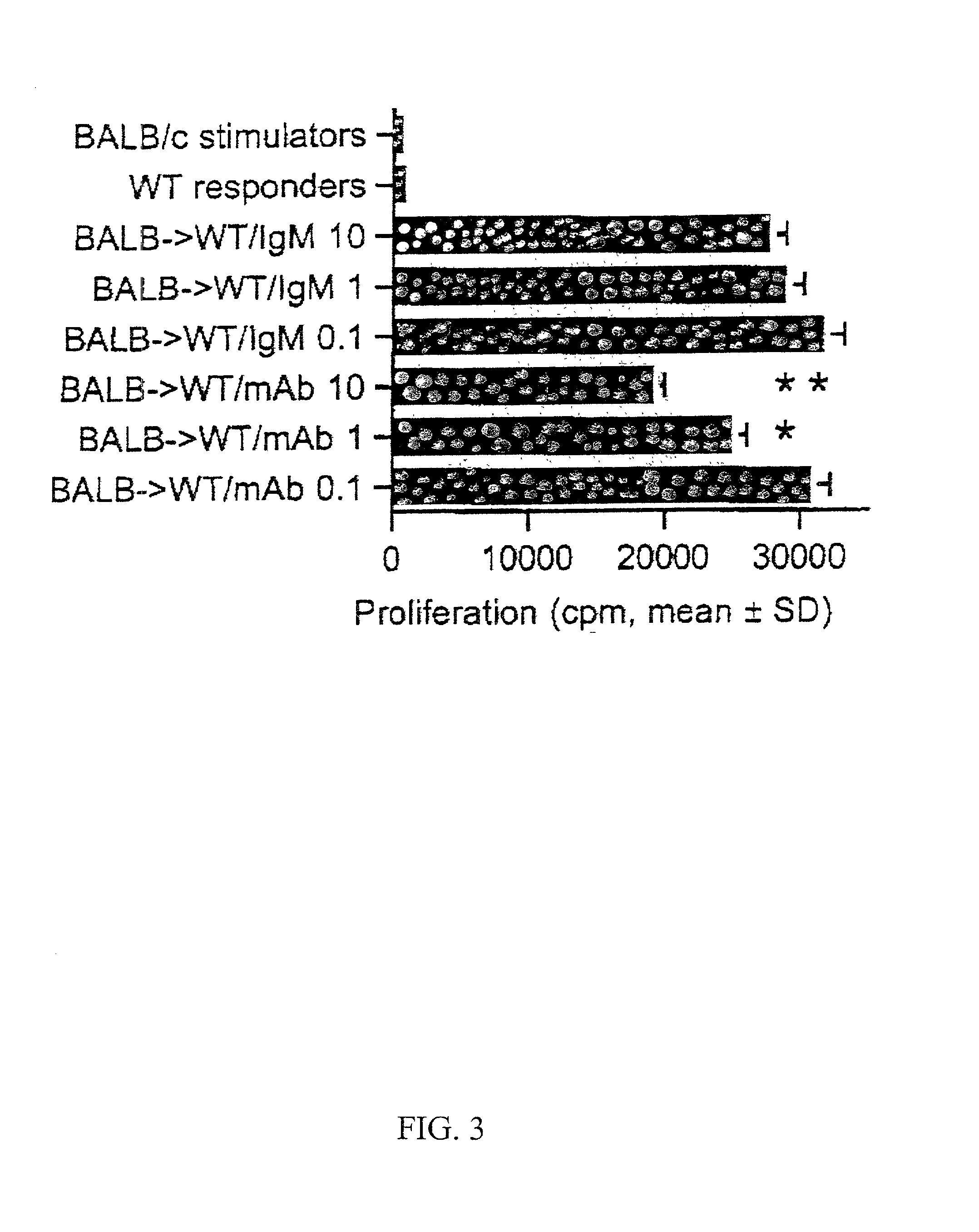
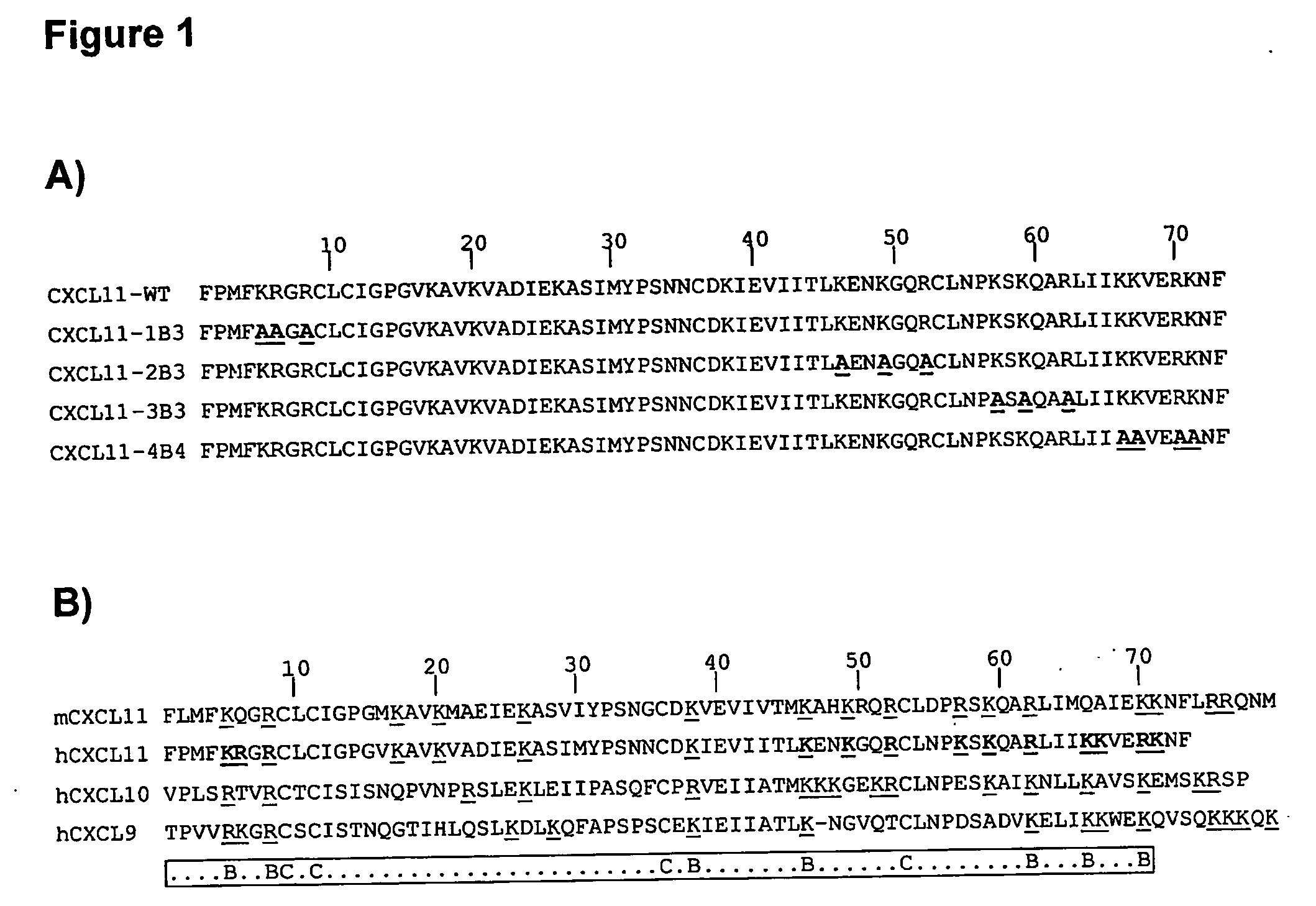
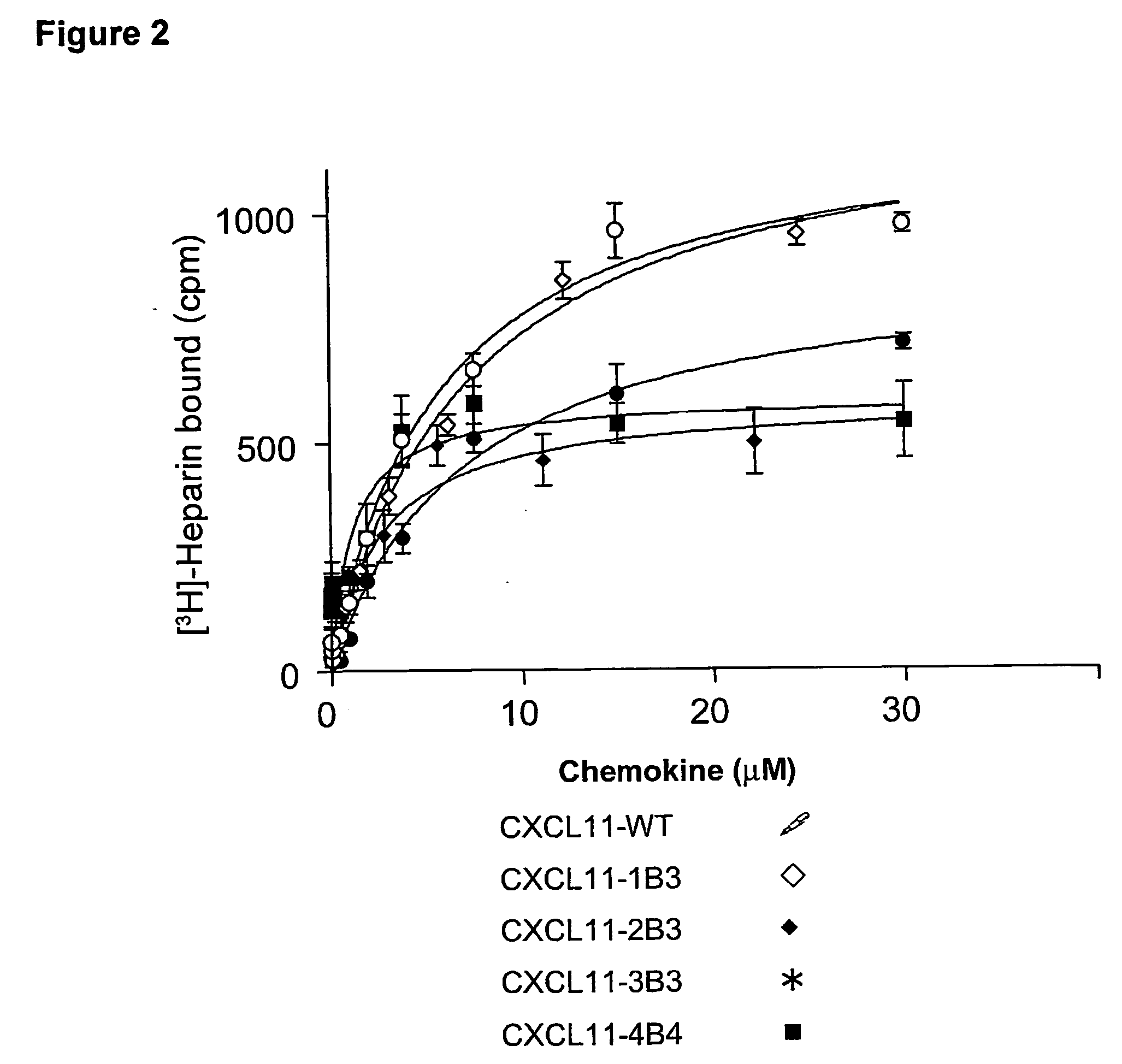
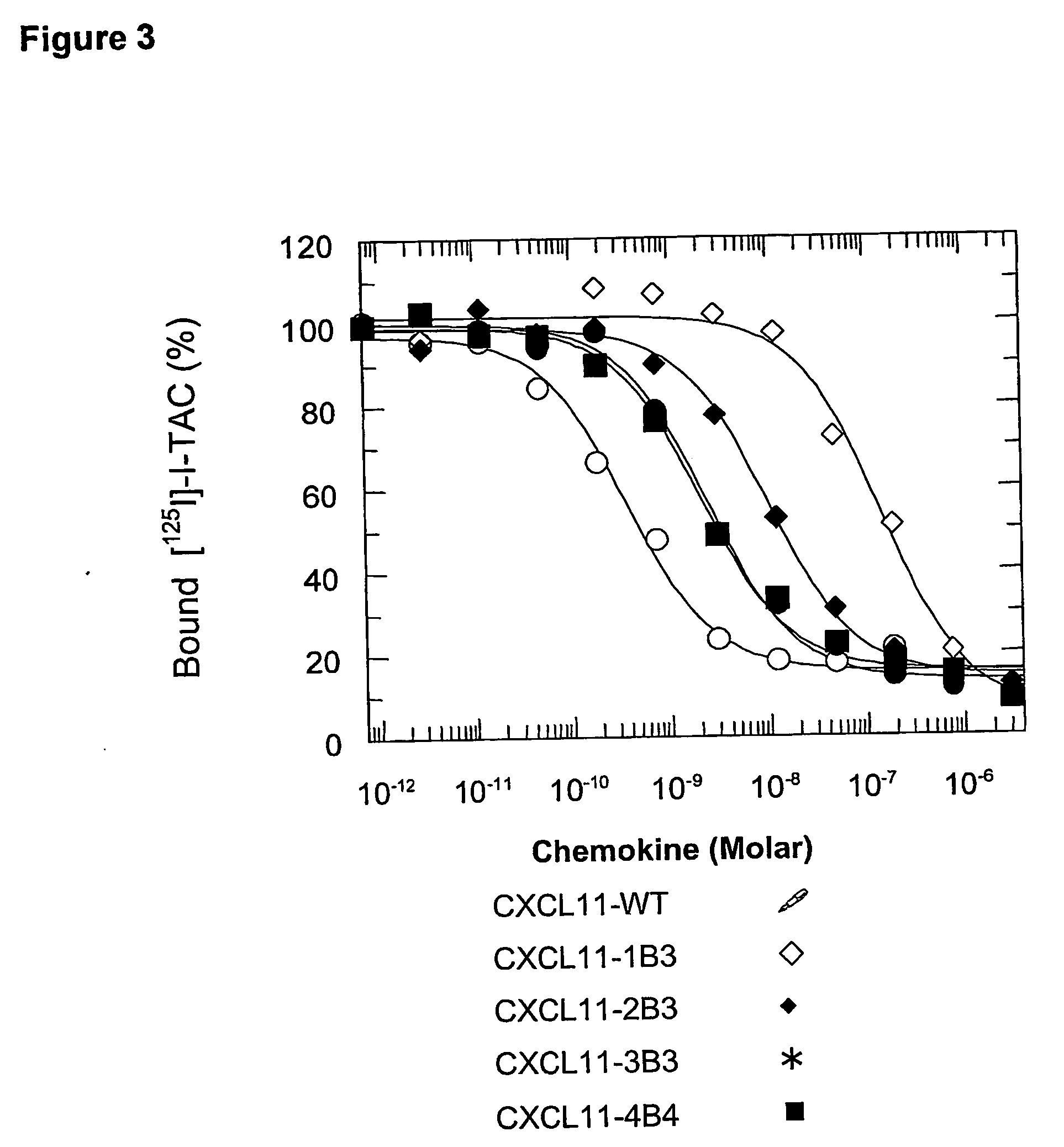
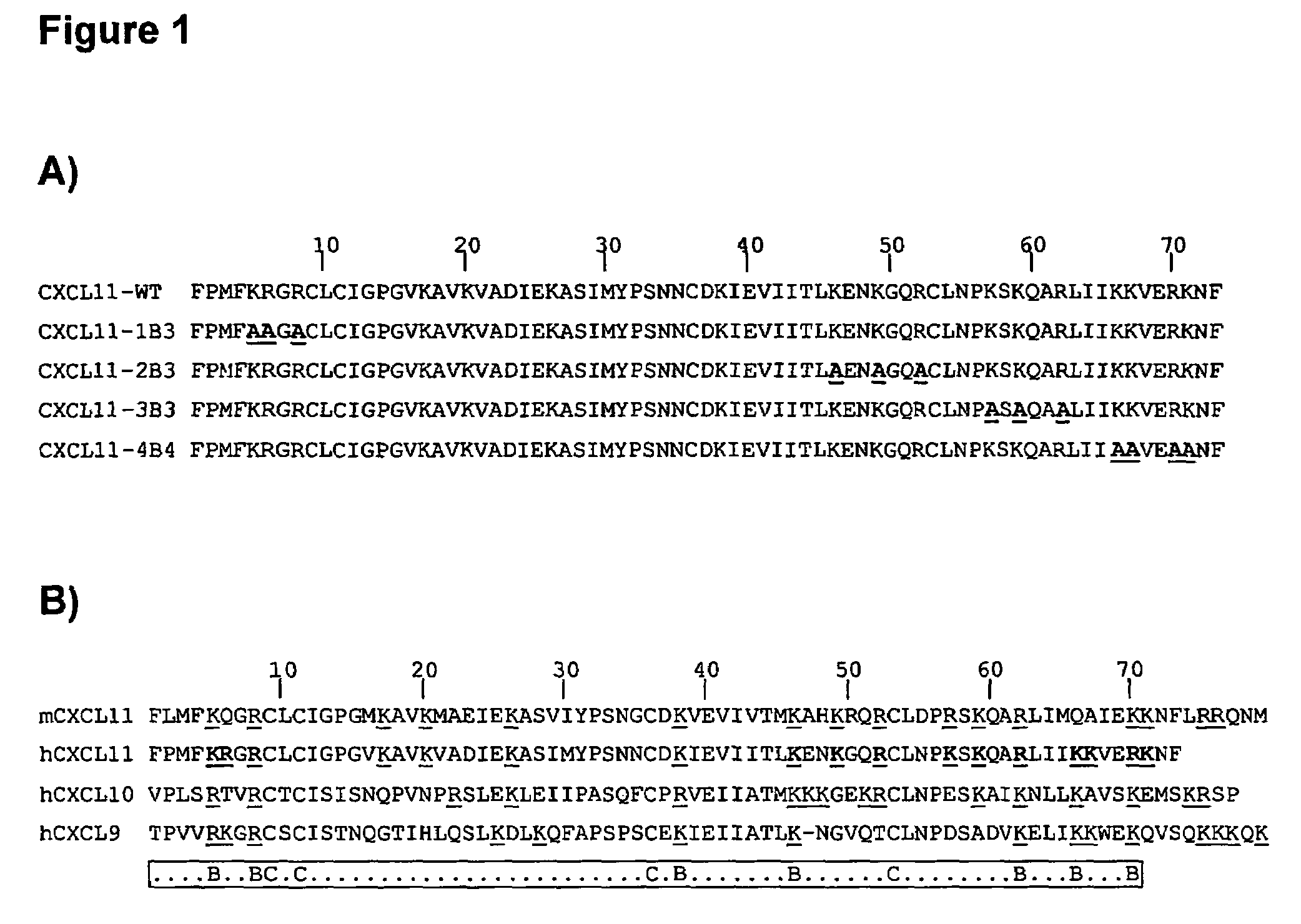
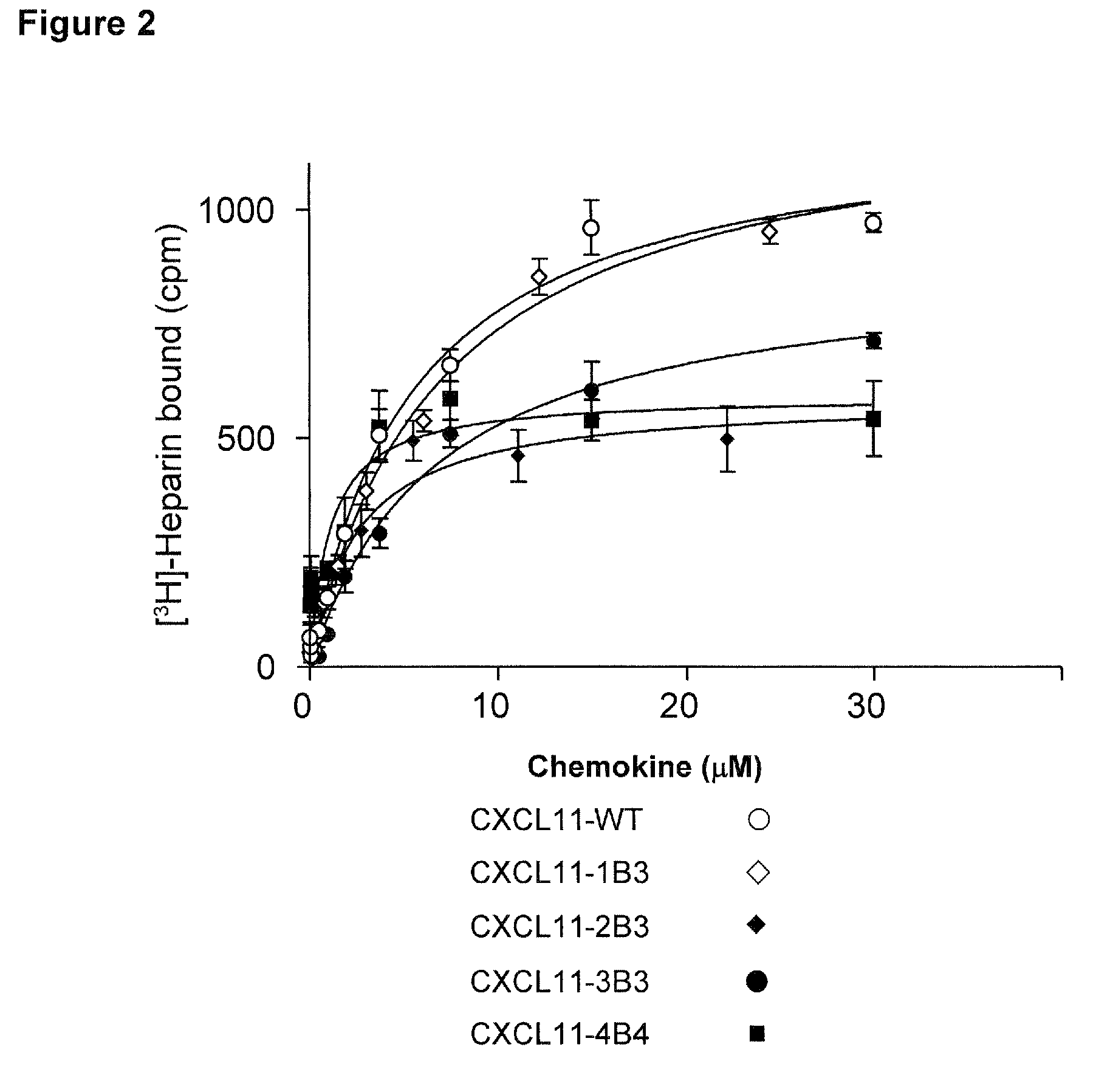
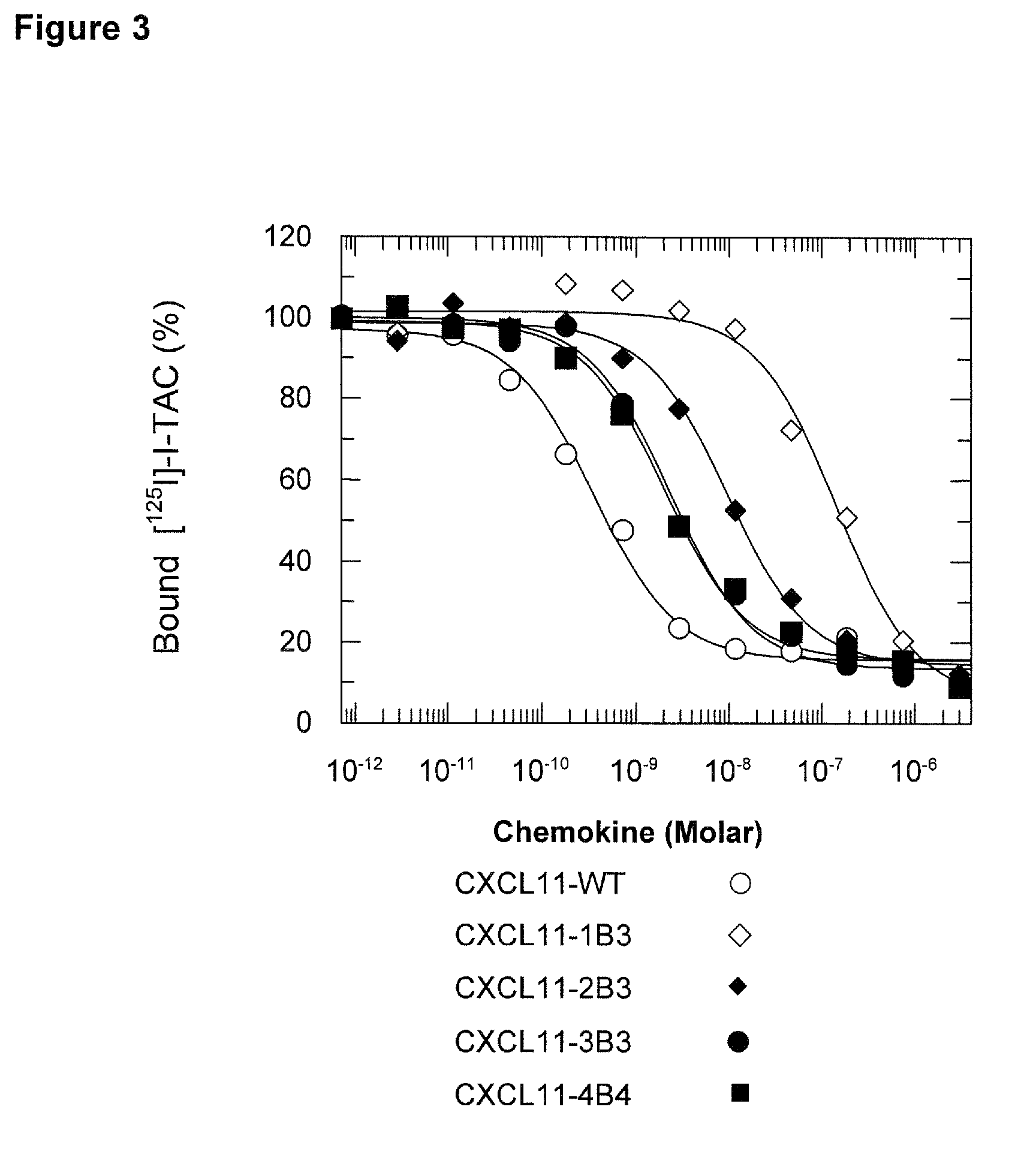
Novel antagonists of CXCR3-binding CXC chemokines, and in particular of human CXCL11, can be obtained by generating mutants of such chemokines in which the binding to glycosaminoglycans (GAGs) is impaired due to non-conservative substitutions of amino acids involved in this interaction. Compounds prepared in accordance with the present invention can be used to block the activity of CXCR3-binding CXC chemokines on CXCR3-expressing cells, thereby providing therapeutic compositions for use in the treatment or prevention of diseases related to excessive activated T cells migration, such as graft rejection and autoimmune diseases, and of diseases needing an increase of vascularization, such as ischemic heart disease.
Owner:MERCK SERONO SA
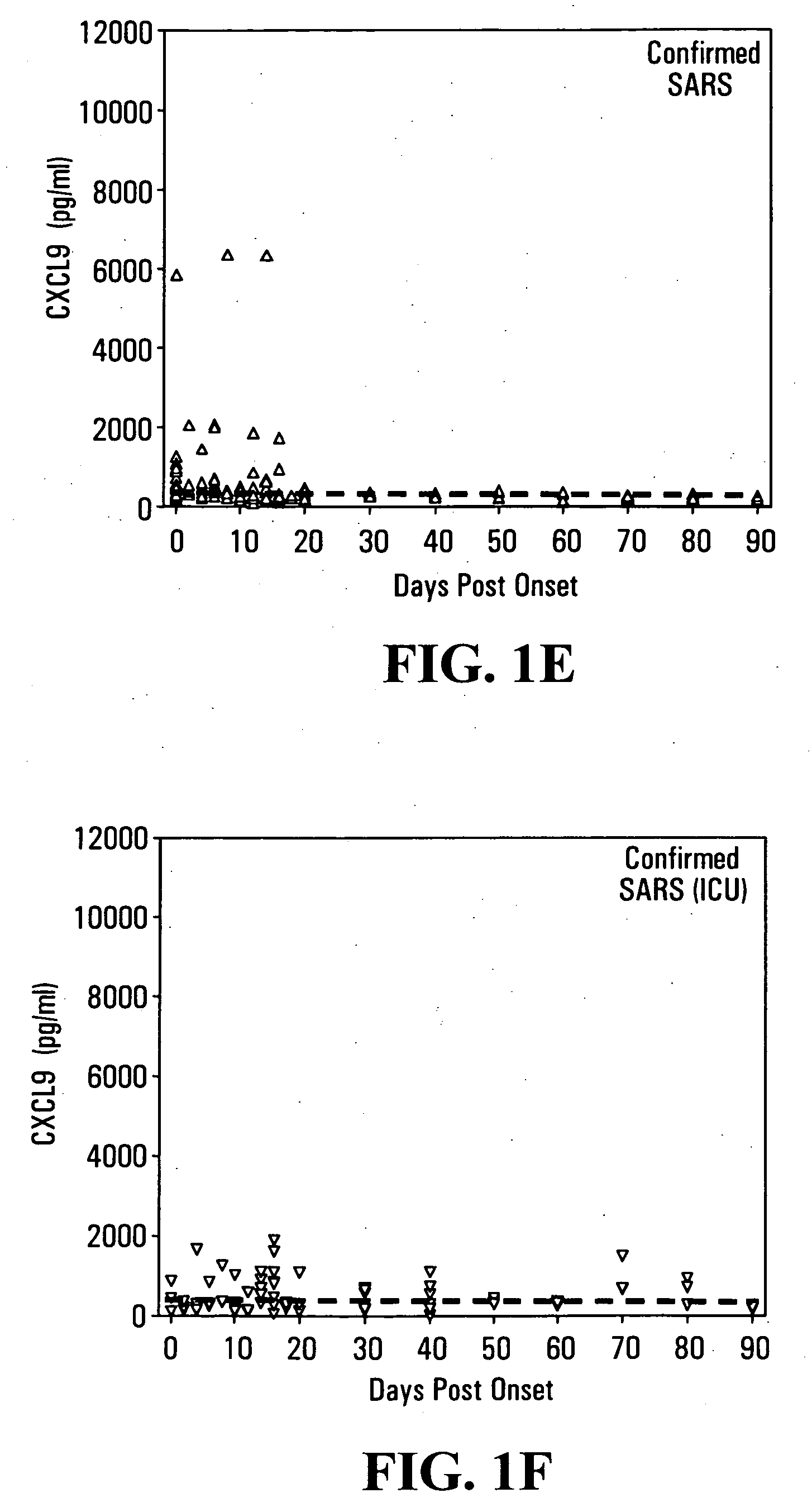
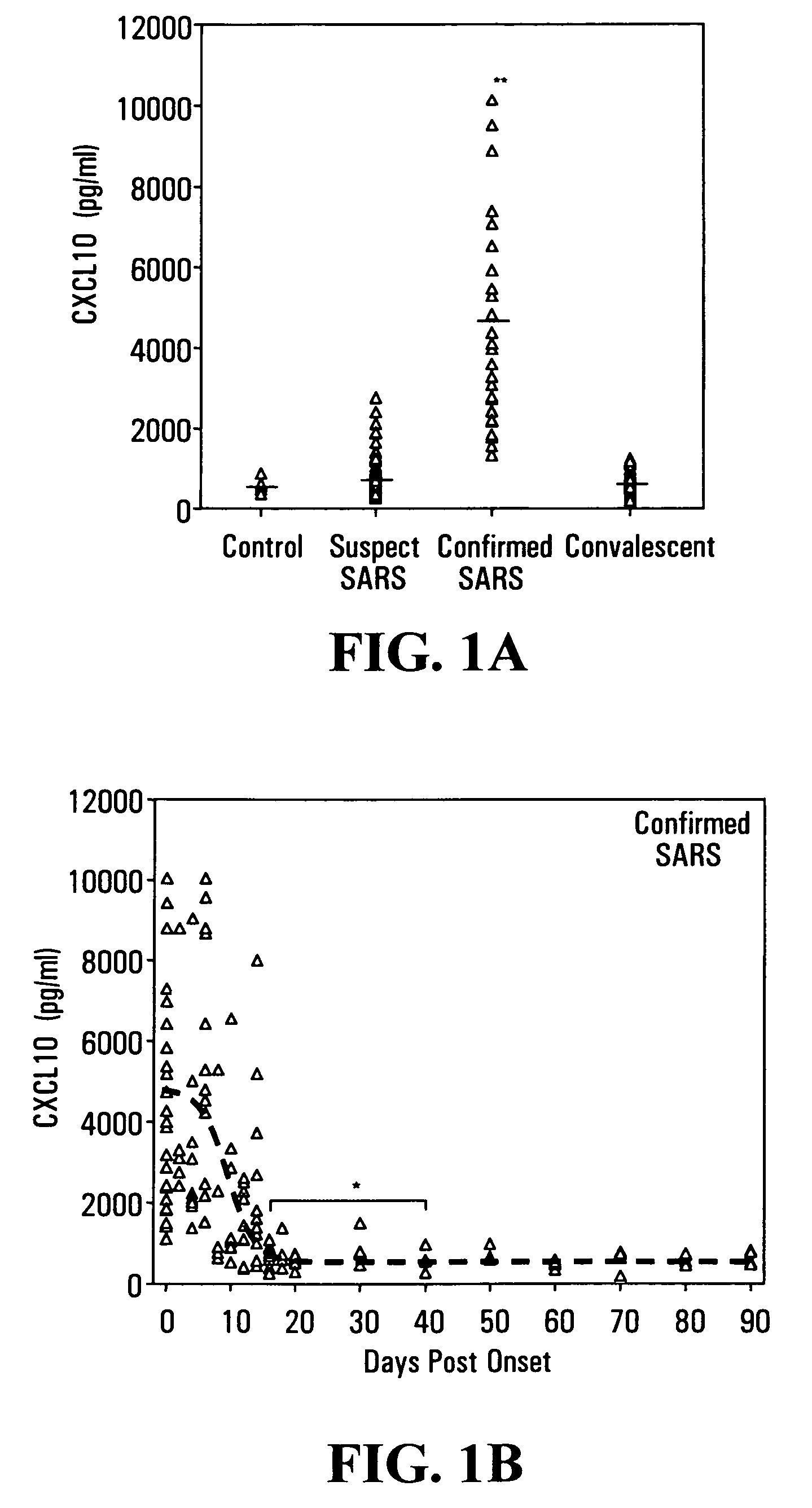
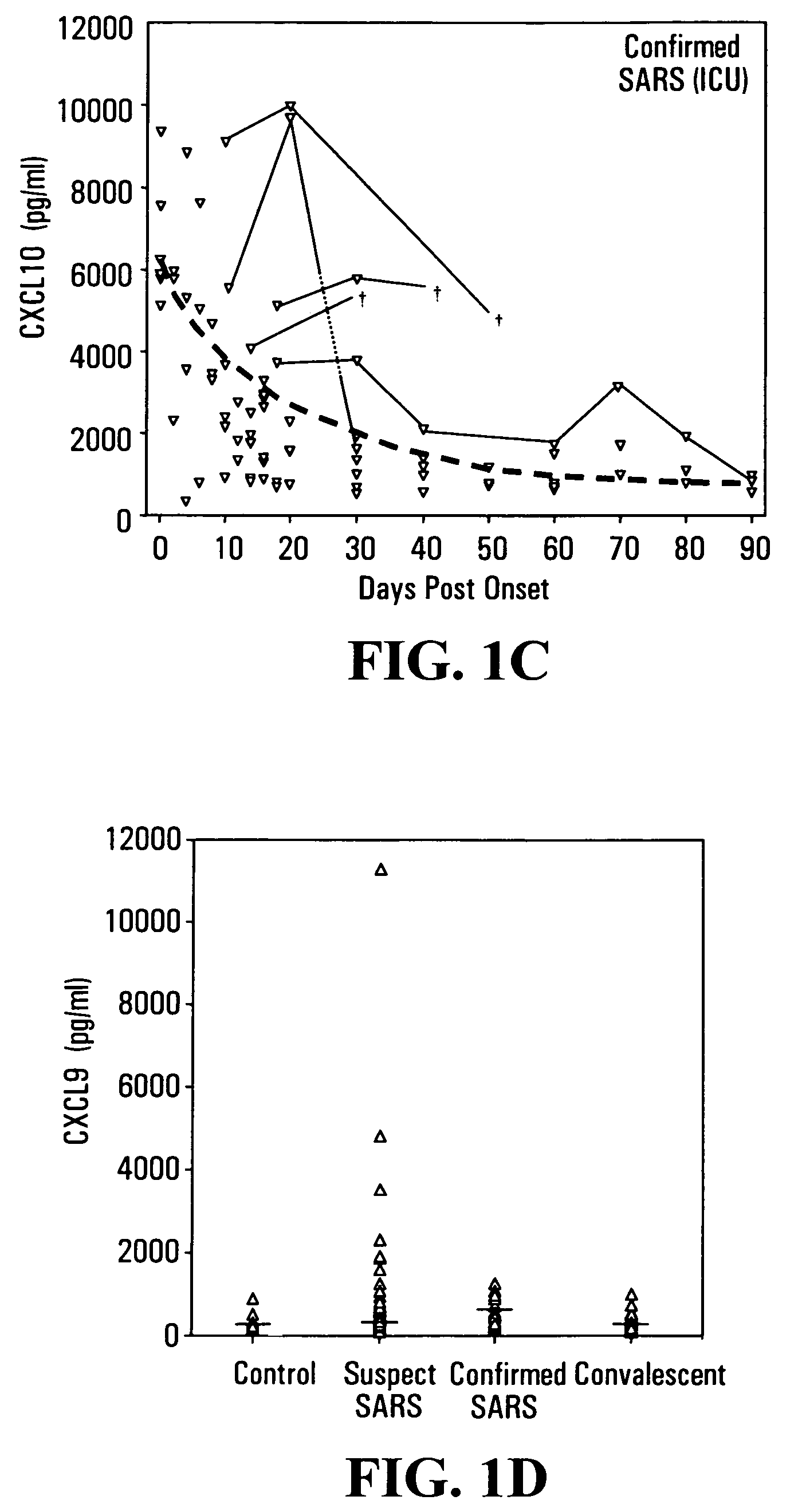
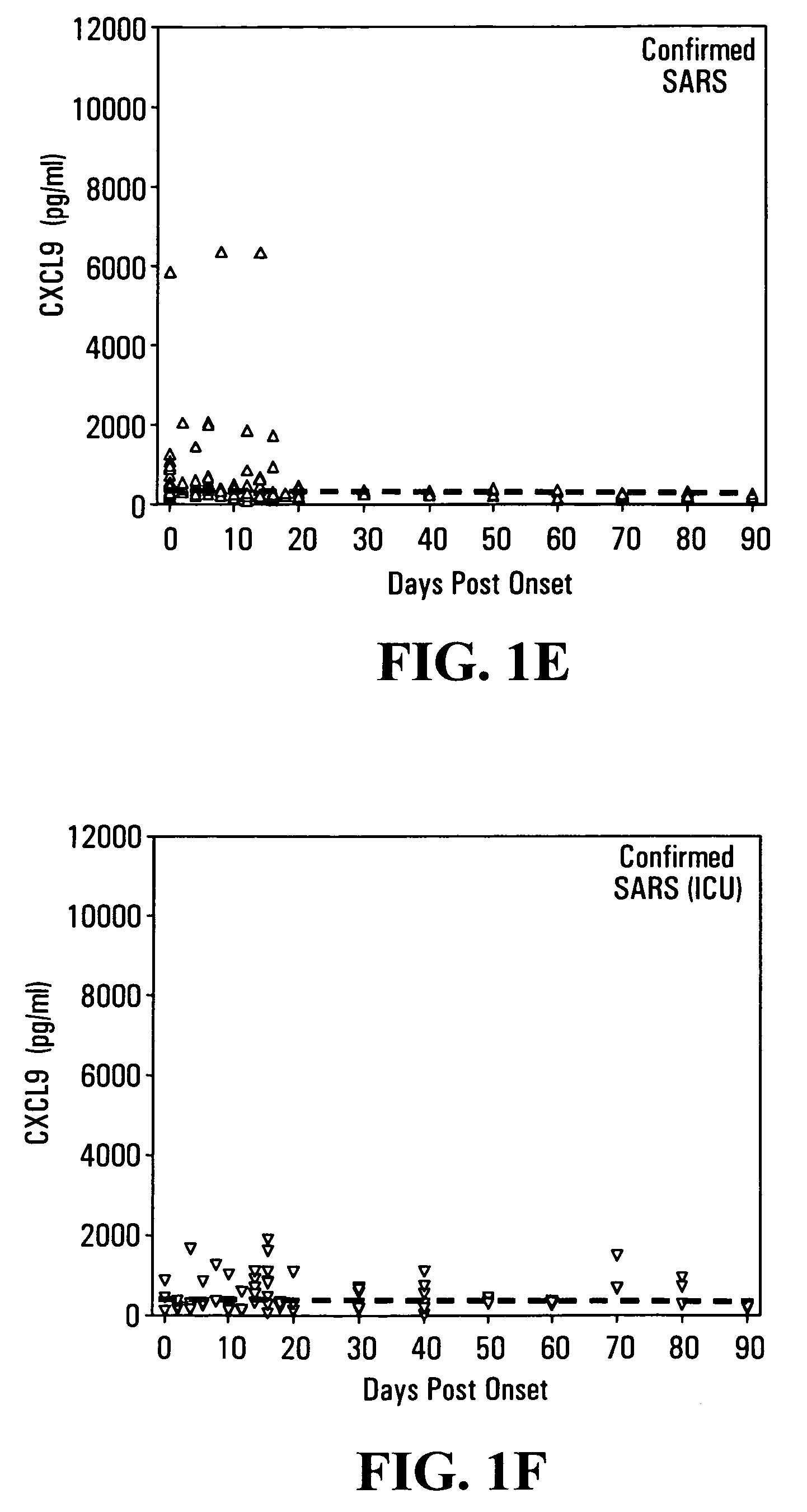
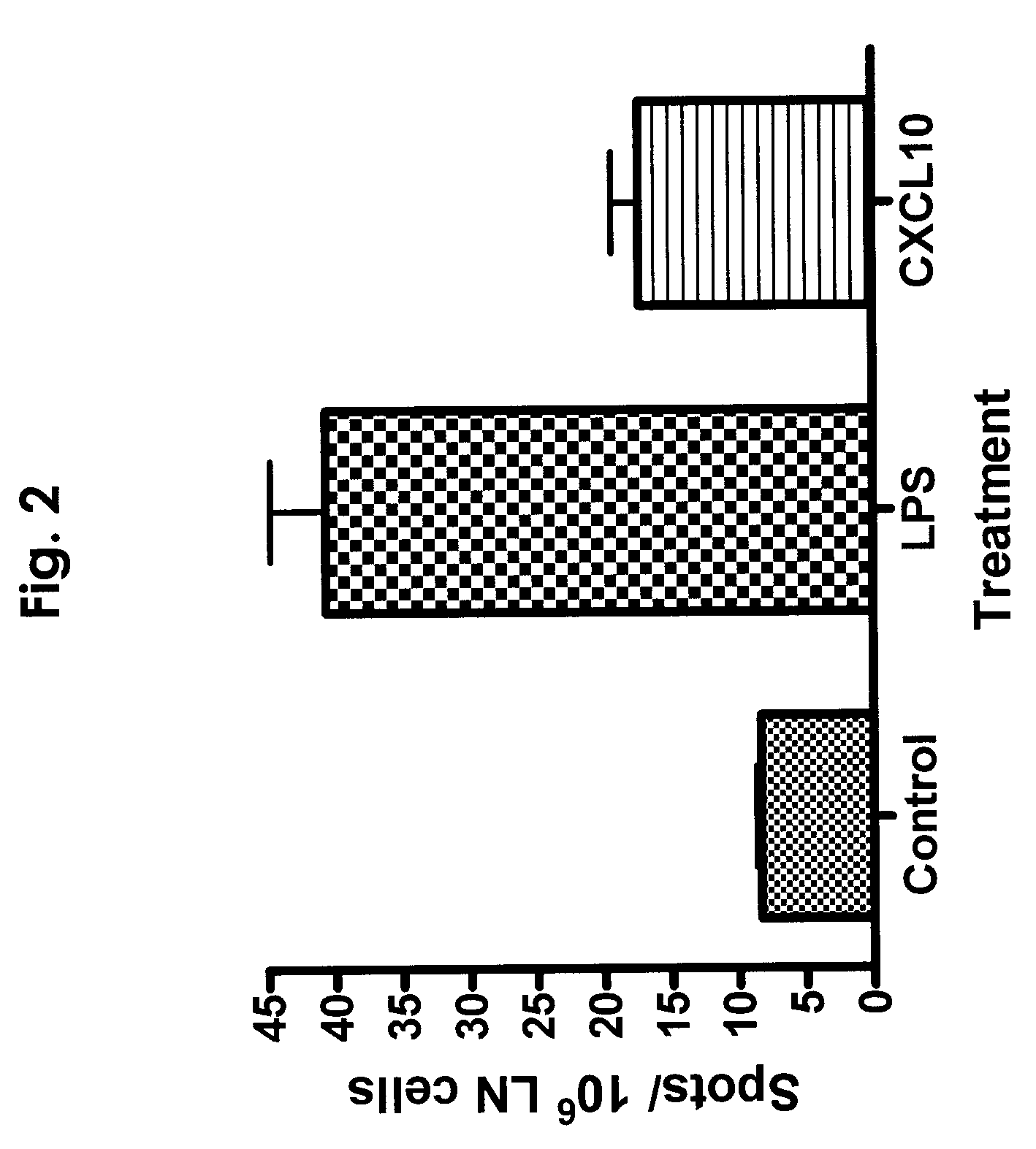
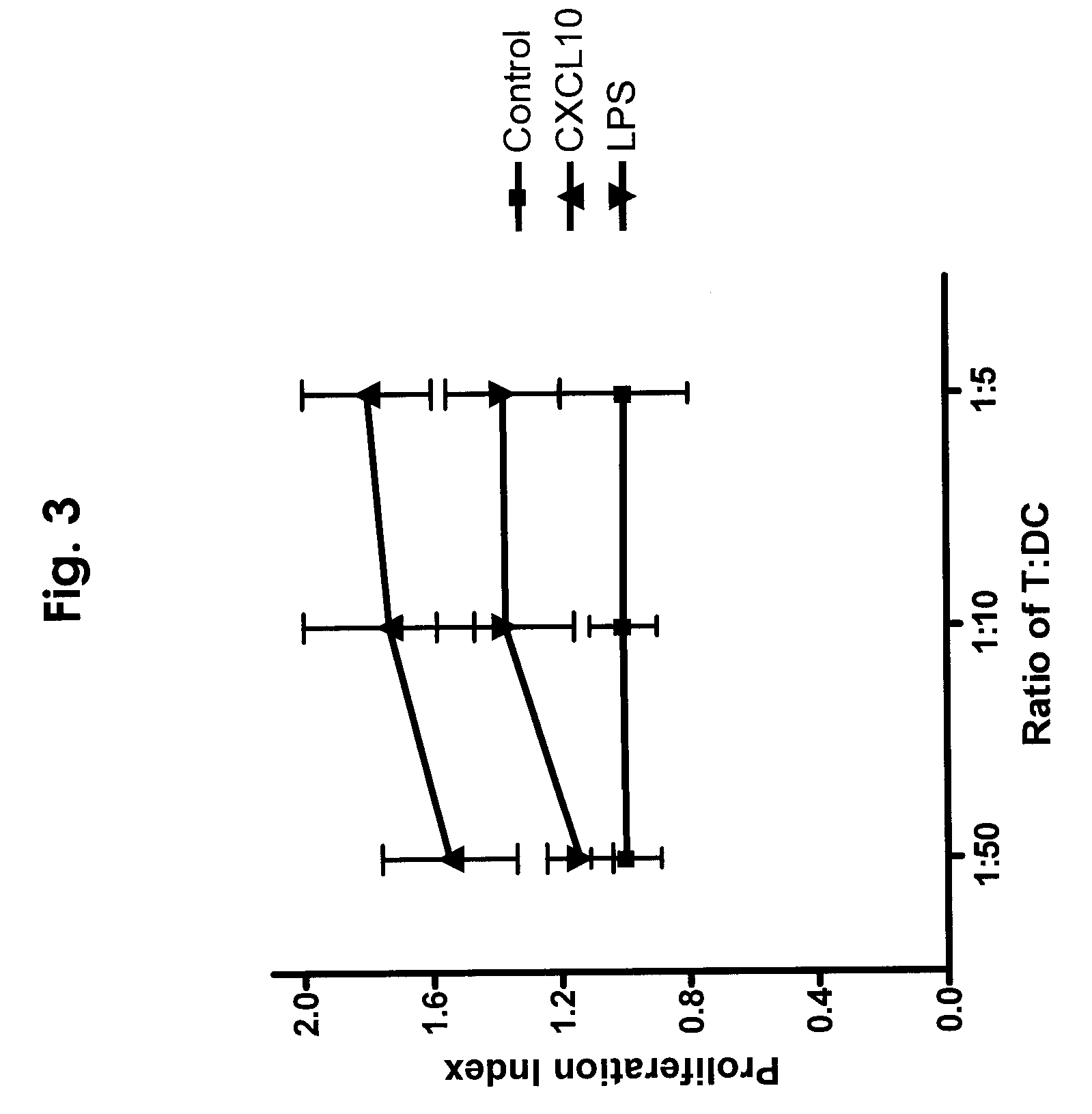
CXCL10-based diagnosis and treatment of respiratory illnesses
InactiveUS20060040329A1In-vivo radioactive preparationsMicrobiological testing/measurementDiseaseCXCL10
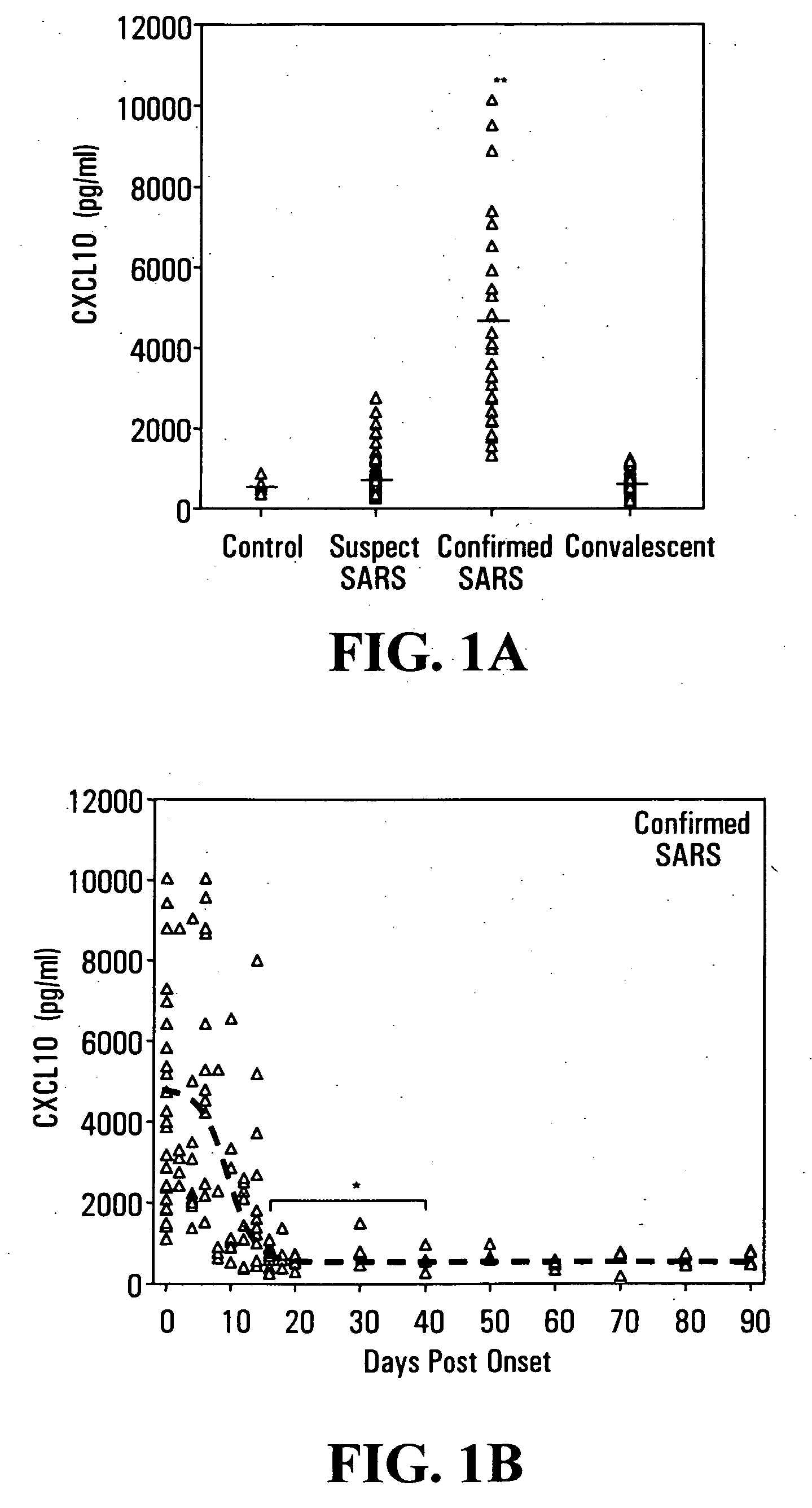
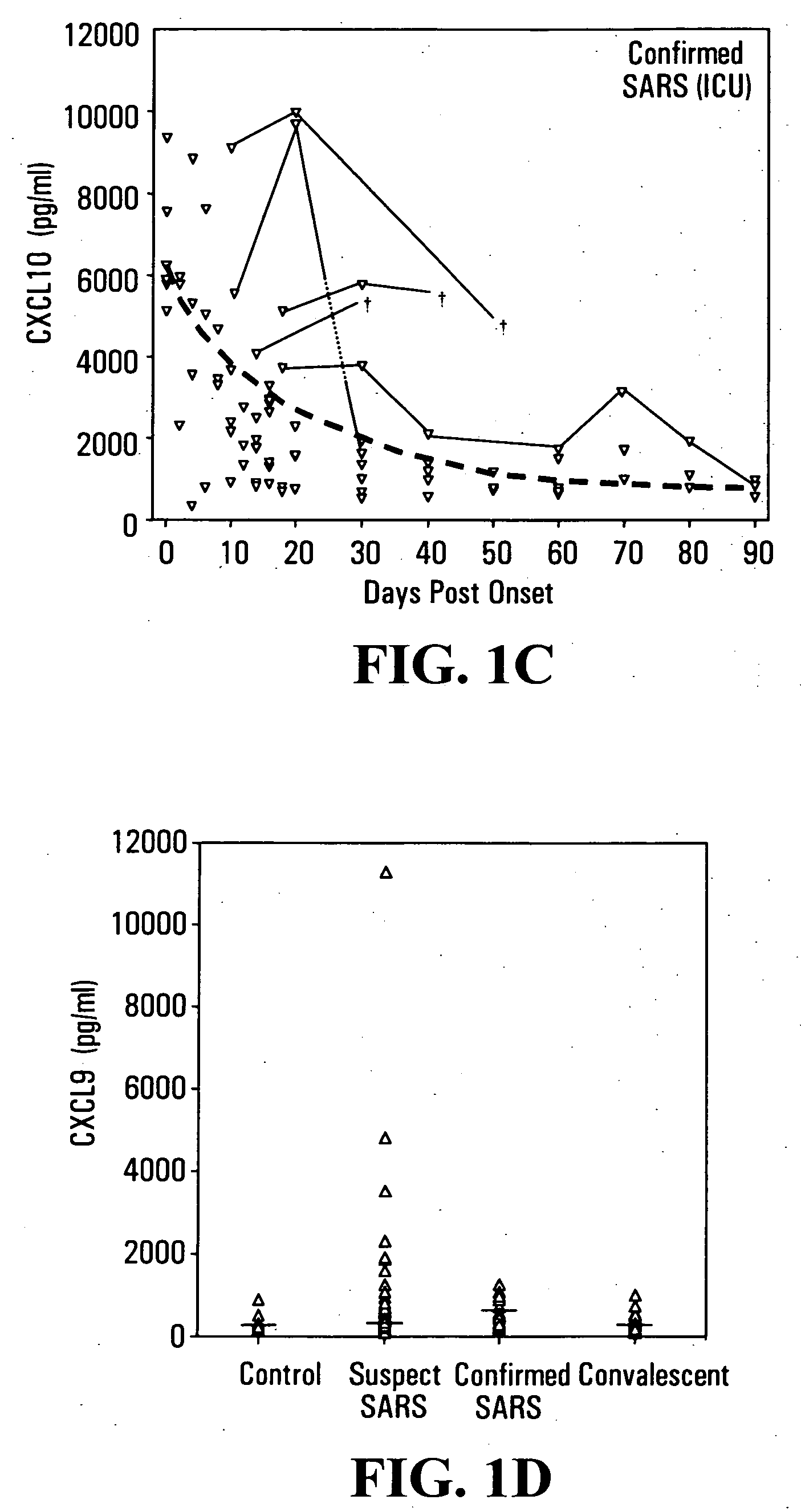
Elevated blood levels of the chemokine CXCL10 polypeptide are associated with respiratory illnesses (e.g. SARS, influenza and community-acquired pneumonia) and are useful in diagnosis of patients. Methods are provided for diagnosis and treatment of patients suffering from respiratory illnesses. Methods are provided for identifying inhibitors of the CXCL10:CXCR3 axis, for use in treating patients suffering from respiratory illnesses.
Owner:UNIV HEALTH NETWORK
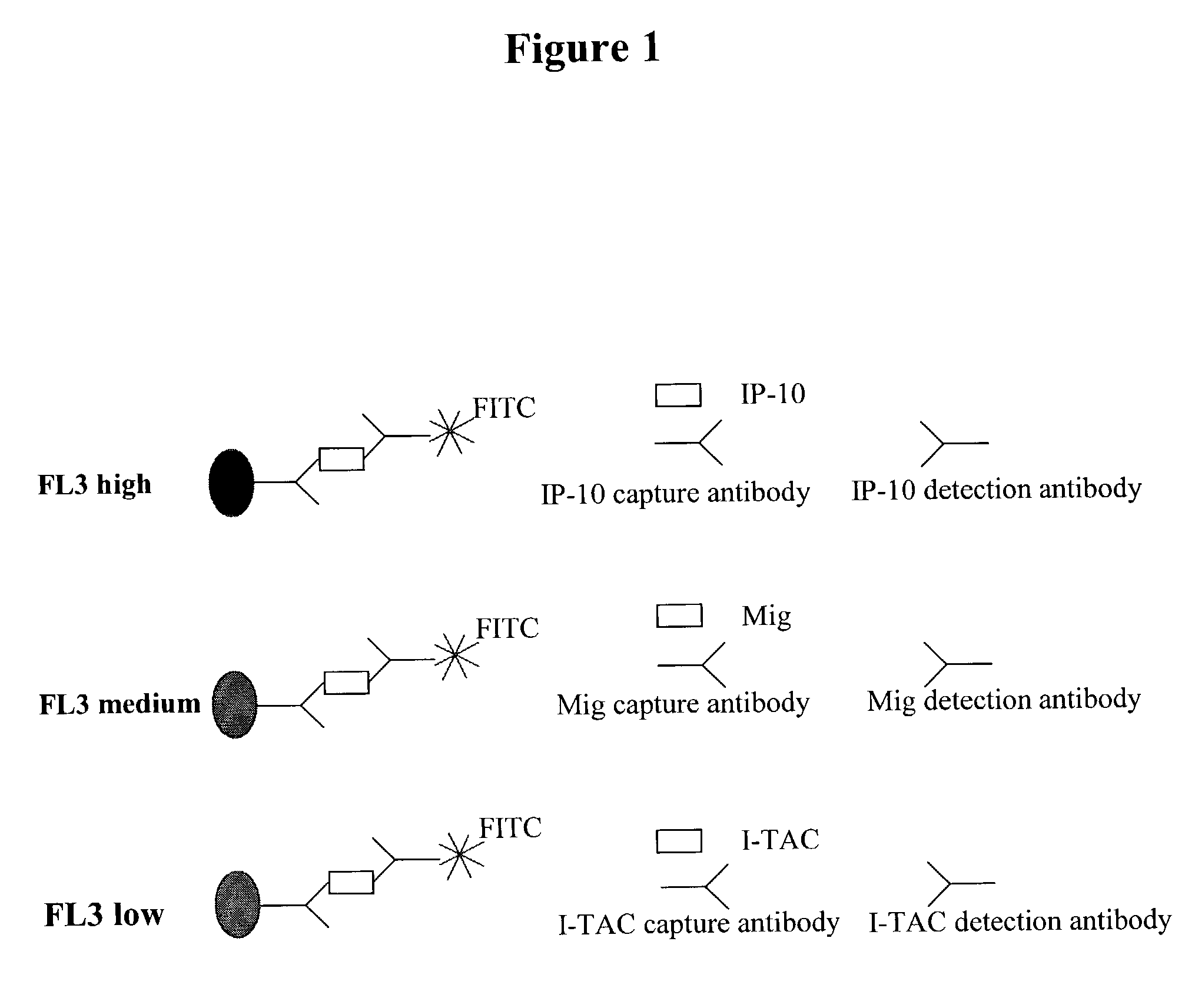
Kit and method for comprehensively evaluating functions of immune cells in human peripheral blood
The invention provides a kit and a method for comprehensively evaluating functions of immune cells in human peripheral blood. The kit for comprehensively evaluating the functions of the immune cells in the human peripheral blood comprises an anti-human CD3, CD4, CD8, CD19, CD21, CD24, CD25, CD27, CD28, CD38, CD56, CD57, CD94, CD127, CD45RA, CXCR3, CXCR5, CCR4, CCR6, CCR7, HLA-DR, PD-1, P30, P46, NKG2D, KIR (NKB1), gamma delta, v delta 2, IgD and IgM antibodies, wherein the antibodies carry fluorescein labels. The kit can be used for comprehensively evaluating the functions of the immune cellsin the human peripheral blood, and is convenient and safe to use.
Owner:东莞暨南大学研究院
Cxcr3 receptor antagonists
Owner:BOEHRINGER INGELHEIM INT GMBH
Method of inhibiting leukocytes with human cxc chemokine receptor 3 antibody
InactiveUS20100061983A1Sugar derivativesPeptide/protein ingredientsExocytosisAntiendomysial antibodies
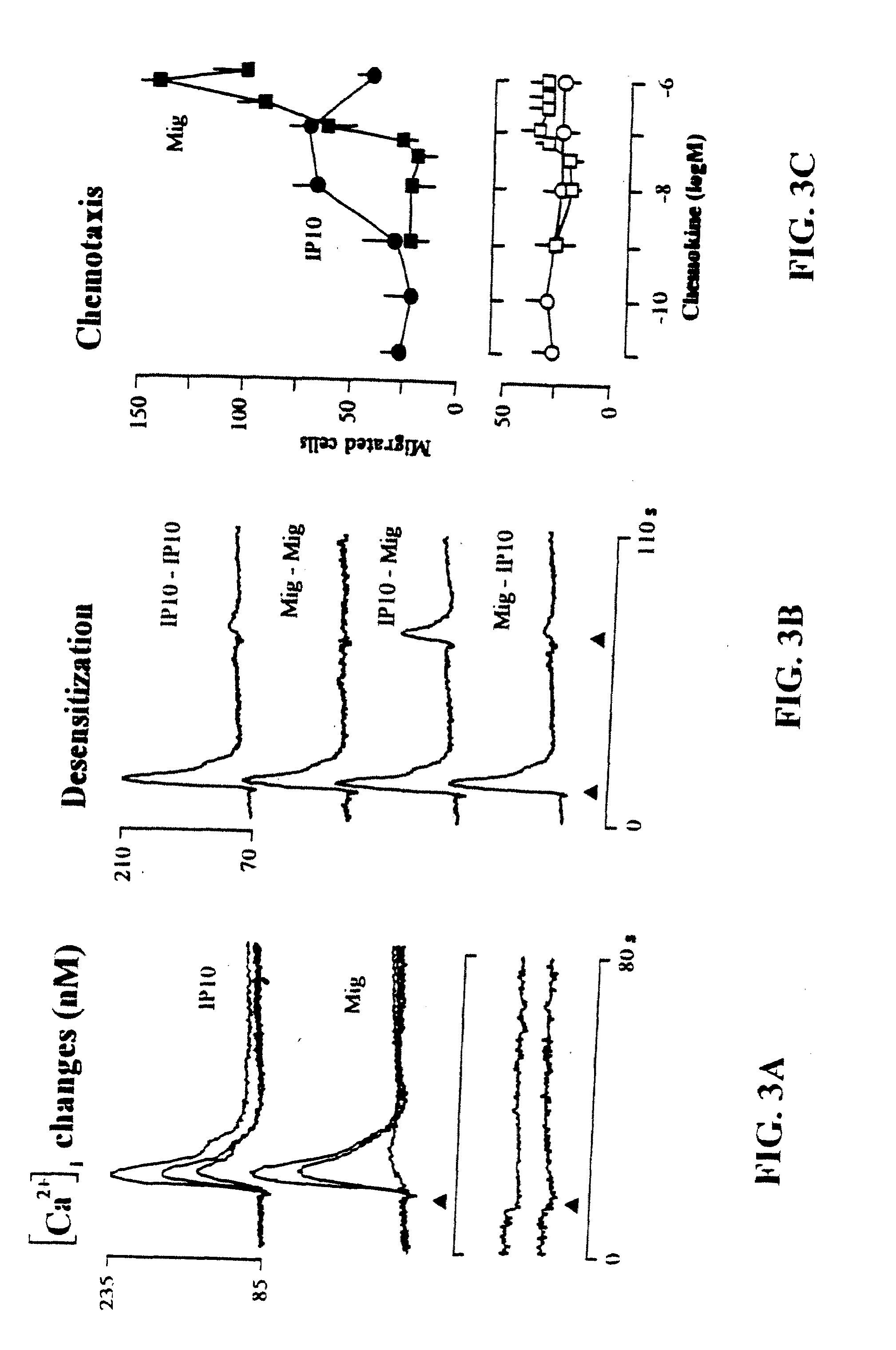
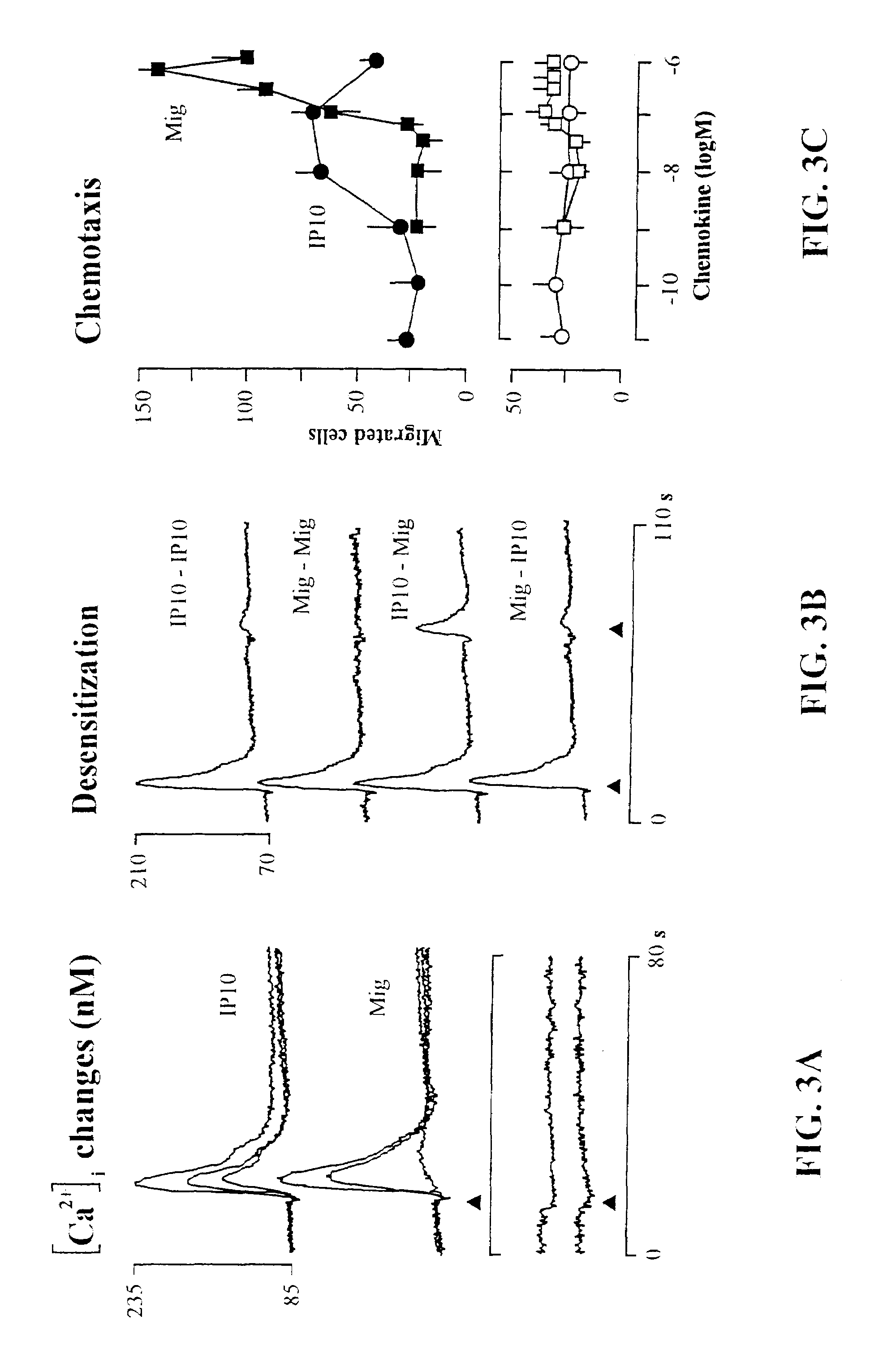
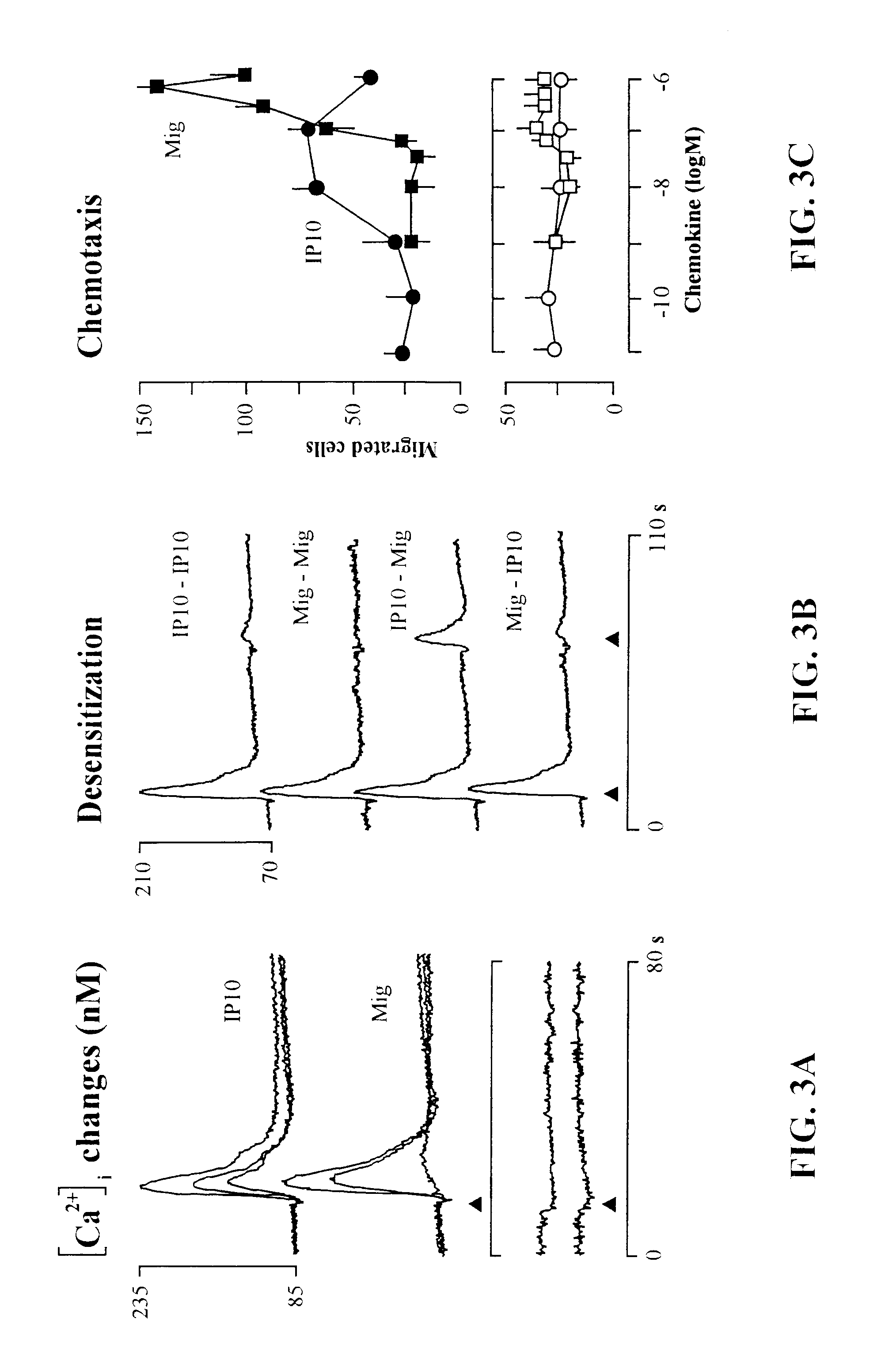
The present invention relates to proteins or polypeptides, referred to herein as isolated and / Or recombinant mammalian (e.g., human) IP-10 / Mig receptor proteins designated CXC Chemokine Receptor 3 (CXCR3) and variants thereof, including those characterized by selective binding of one or more chemokines (e.g., IP-10 and / or Mig), and / or the ability to induce a cellular response (e.g., chemotaxis, exocytosis). Antibodies reactive with CXCR3 receptors can be produced using the proteins or variants thereof or host cells comprising same as immunogen.
Owner:THEODOR KOCHER INST +1
Method of inhibiting leukocytes with human CXC chemokine receptor 3 antibody
The present invention relates to proteins or polypeptides, referred to herein as isolated and / or recombinant mammalian (e.g., human) IP-10 / Mig receptor proteins designated CXC Chemokine Receptor 3 (CXCR3) and variants thereof, including those characterized by selective binding of one or more chemokines (e.g., IP-10 and / or Mig), and / or the ability to induce a cellular response (e.g., chemotaxis, exocytosis). Antibodies reactive with CXCR3 receptors can be produced using the proteins or variants thereof or host cells comprising same as immunogen.
Owner:MILLENNIUM PHARMA INC +1
Cxcr3 receptor antagonists
Owner:BOEHRINGER INGELHEIM INT GMBH
Immune function assessment kit and assessment method for autoimmune disease patients
InactiveCN109406775AEasy to useOperational securityMaterial analysisAutoimmune thyroid diseaseAutoimmune disease
The invention provides an immune function assessment kit and assessment method for patients with an autoimmune disease. The immune function assessment kit for the patients the autoimmune disease comprises anti-human CD3, CD4, CD8, CD19, CD21, CD24, CD25, CD27, CD28, CD38, CD57, CD127, CD45RA, CXCR3, CXCR5, CCR4, CCR6, CCR7, HLA-DR, PD-1, IgD, and IgM antibodies, and all antibodies referred carry fluorescein labels. The kit can be used for carrying out comprehensive assessment to the function of immune cells of the patients with the autoimmune disease, and usage is convenient and safe.
Owner:东莞市暨科生物科技有限公司
Anti-CXCL9, anti-CXCL 10, anti-CXCL 11, anti-CXCL 13, anti-CXCR3 and anti-CXCR5 agents for inflammatory disorder
A method for detecting an inflammatory disease in a subject is disclosed. The method comprises the steps of (a) detecting a level of expression of one or more inflammatory disease markers in a biological sample obtained from the subject; and (b) comparing the level of expression of said one or more inflammatory disease markers in the biological sample to a normal level of expression of the one or more inflammatory disease markers, wherein the one or more inflammatory disease markers comprise one or more markers selected from the group consisting of CXCL9, CXCLIO, CXCLl 1, CXCLl 3, CXCR3 and CXCR5. Also disclosed are a method for monitoring the course of treatment for an inflammatory disease in a subject and a kit for detecting an inflammatory disease in a subject.
Owner:JYANT TECH
Novel blood sugar controller and method of screening the same
InactiveUS20050119174A1Impaired glucose tolerancePromote insulin secretionPeptide/protein ingredientsImmunoglobulinsDiseaseScreening method
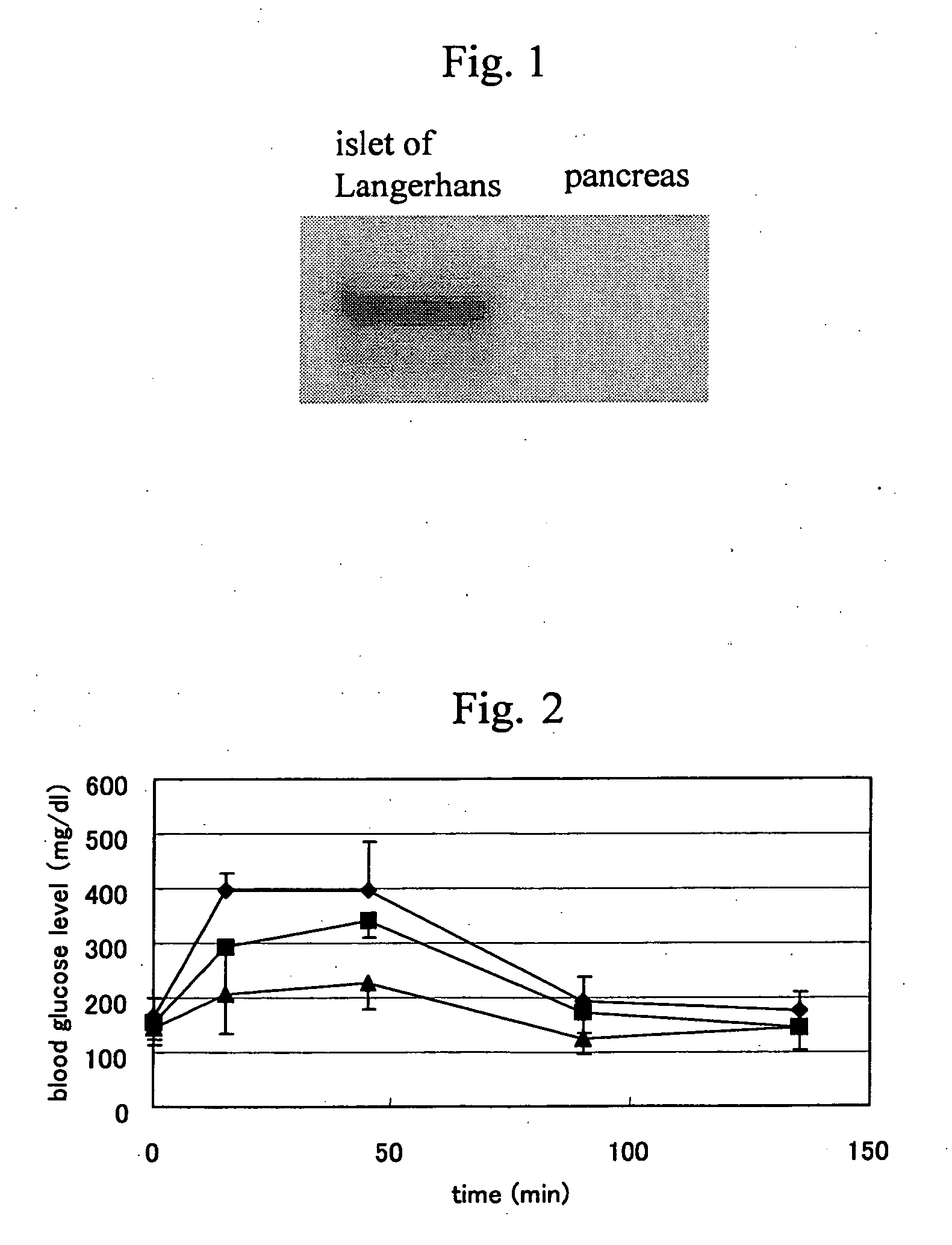
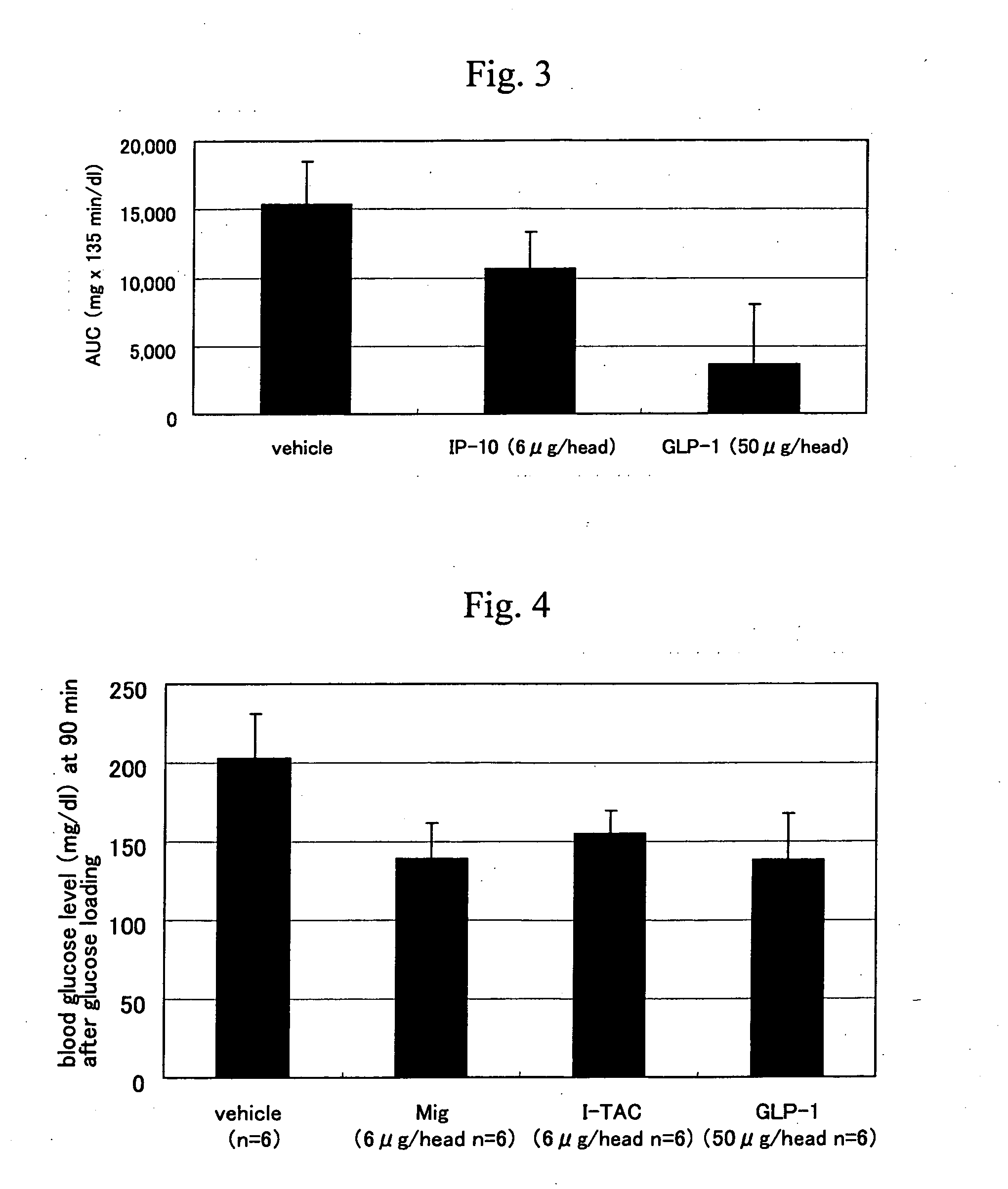
The present invention provides an impaired glucose tolerance ameliorating drug and a therapeutic drug for lifestyle-related diseases, particular an antidiabetic drug, which contain a CXCR3 agonist as an active ingredient; a hypoglycemia ameliorating drug, a therapeutic drug for insulinoma or an anti-obesity drug containing a CXCR3 antagonist as an active ingredient; a method of screening for a new CXCR3 ligand using CXCR3 and a known CXCR3 ligand; a method of screening a CXCR3 ligand, which uses a co-expression system of CXCR3 and a coupling G protein; and a diagnostic method for type II diabetes, which includes detecting an amount of a CXCR3 ligand expressed in a biological sample.
Owner:SUMITOMO DAINIPPON PHARMA CO LTD
Methods for predicting the survival time and treatment responsiveness of a patient suffering from a solid cancer with a signature of at least 7 genes
ActiveUS20150203919A1Improve efficiencyEliminate side effectsBiocideMicrobiological testing/measurementCCL2Good prognosis
The present invention relates to a method for predicting the survival time of a patient suffering from a solid cancer comprising i) determining in a tumor sample obtained from the patient the gene expression level of at least 7 genes selected from the group consisting of CCR2, CD3D, CD3E, CD3G, CD8A, CXCL10, CXCL11, GZMA, GZMB, GZMK, GZMM, IL15, IRF1, PRF1, STAT1, CD69, ICOS, CXCR3, STAT4, CCL2, and TBX21, ii) comparing every expression level determined at step i) with their predetermined reference value and iii) providing a good prognosis when all expression levels determined at step i) are higher than their predetermined reference values, or providing a bad prognosis when all expression levels determined at step i) are lower than their predetermined reference values or providing an intermediate prognosis when at least one expression level determined value is higher than its predetermined value. The method is also particularly suitable for predicting the responsiveness of the patient to a treatment.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +2
CXCL10-based diagnosis and treatment of respiratory illnesses
InactiveUS7332294B2In-vivo radioactive preparationsMicrobiological testing/measurementCXCL10Community-acquired pneumonia
Elevated blood levels of the chemokine CXCL10 polypeptide are associated with respiratory illnesses (e.g. SARS, influenza and community-acquired pneumonia) and are useful in diagnosis of patients. Methods are provided for diagnosis and treatment of patients suffering from respiratory illnesses. Methods are provided for identifying inhibitors of the CXCL10:CXCR3 axis, for use in treating patients suffering from respiratory illnesses.
Owner:UNIV HEALTH NETWORK
Systems and methods for characterizing kidney diseases
InactiveUS7138229B2Microbiological testing/measurementDisease diagnosisNephrosisInterstitial Disease
Owner:RENOVAR
Inhibitors of the chemokine receptor CxCR3
Owner:SANOFI SA
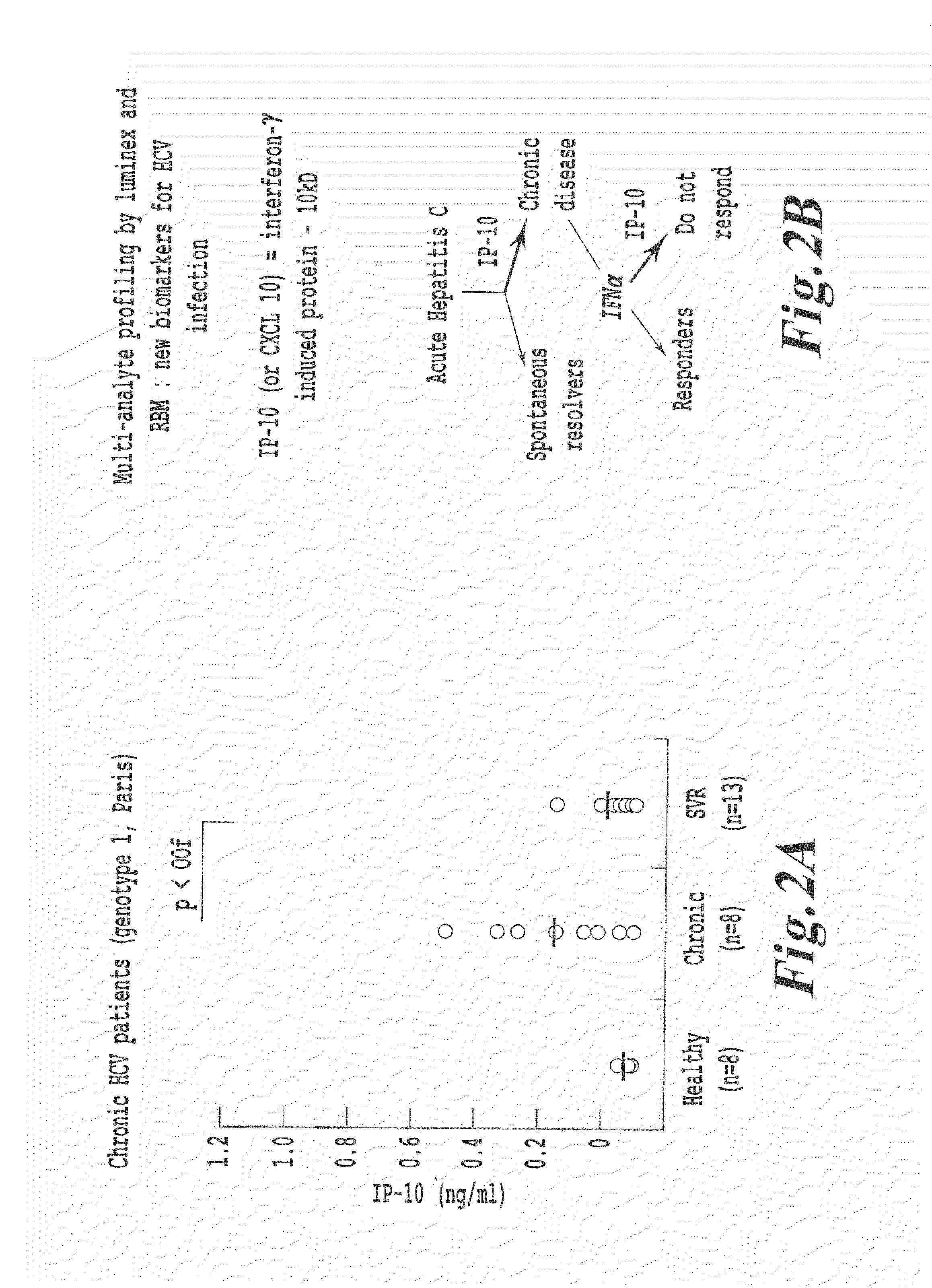
METHODS OF USING sIP-10, CD26 INHIBITORS AND CXCR3 LEVELS IN A SAMPLE TO ASSESS CLEARANCE OF INFECTION, RESPONSE TO INTERFERON THERAPY, AND TREATING CHRONIC INFECTIONS
The present invention relates to methods for the treatment of infection, a disease, or a condition using CD26 (DPIV) inhibitors. The present invention also relates to an antibody that binds to the IP-10 protein and a method of monitoring the necessity for administering a CD26 inhibitor to a patient, comprising evaluating a level of sIP-10, a activity of CD26, and / or a level of CXCR3 cells in a sample.
Owner:INST PASTEUR
Antagonists of cxcr3-binding cxc chemokines
InactiveUS7541435B2Reduced tendency to interactAntibacterial agentsPeptide/protein ingredientsAutoimmune diseaseNon conservative
Novel antagonists of CXCR3-binding CXC chemokines, and in particular of human CXCL11, can be obtained by generating mutants of such chemokines in which the binding to glycosaminoglycans (GAGs) is impaired due to non-conservative substitutions of amino acids involved in this interaction. Compounds prepared in accordance with the present invention can be used to block the activity of CXCR3-binding CXC chemokines on CXCR3-expressing cells, thereby providing therapeutic compositions for use in the treatment or prevention of diseases related to excessive activated T cells migration, such as graft rejection and autoimmune diseases, and of diseases needing an increase of vascularization, such as ischemic heart disease.
Owner:MERCK SERONO SA
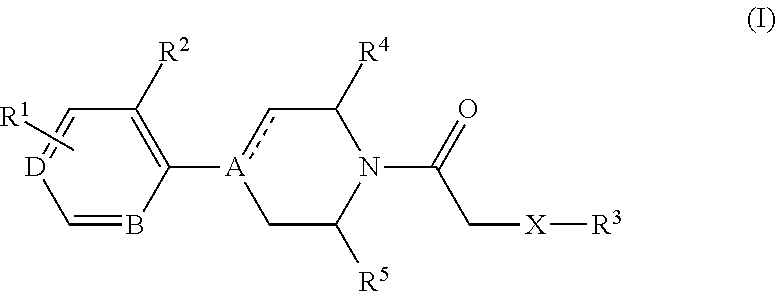
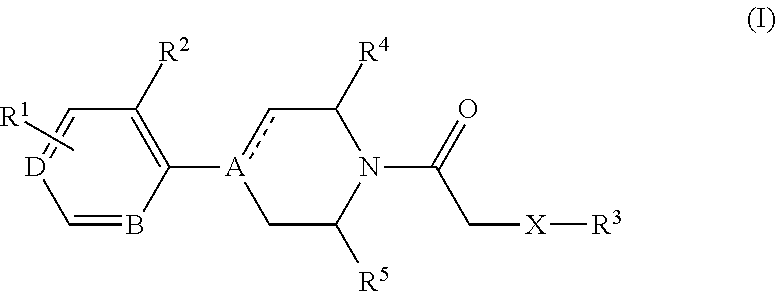
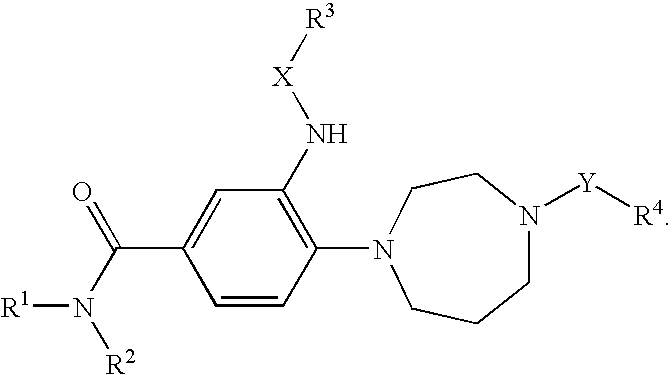
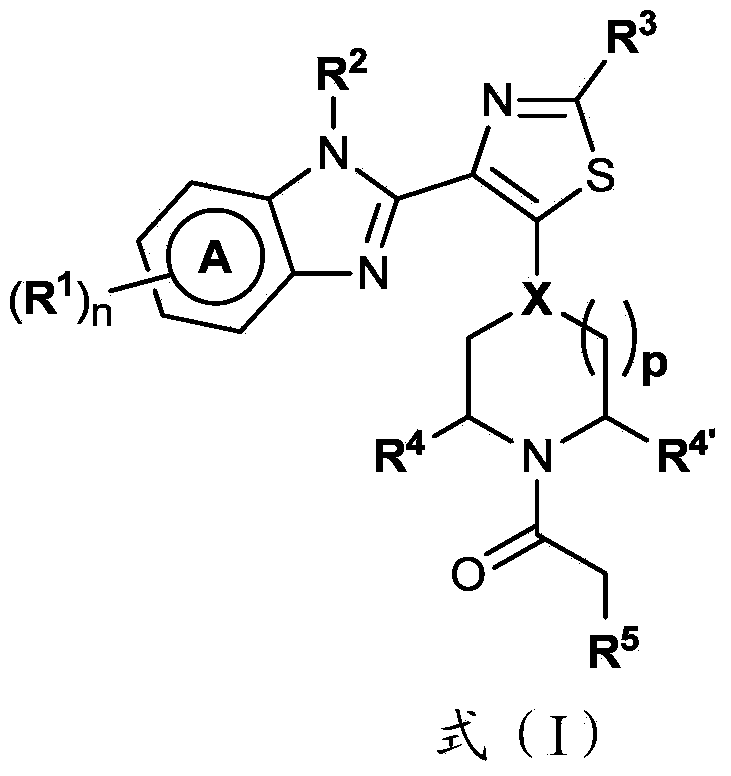
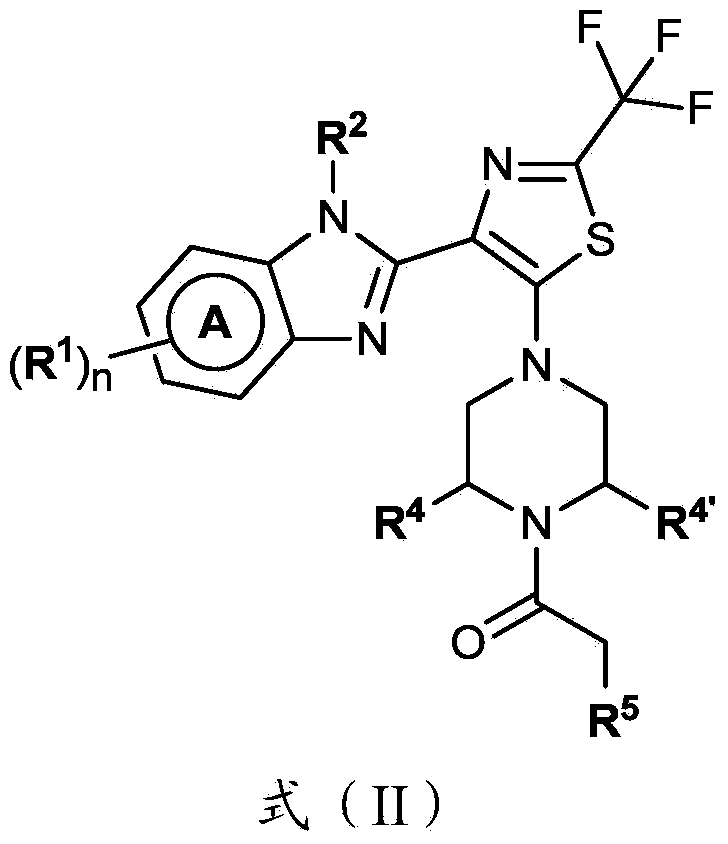
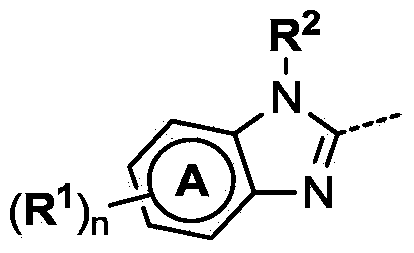
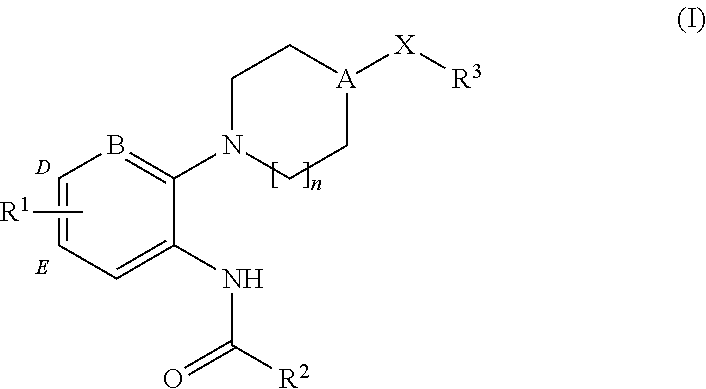
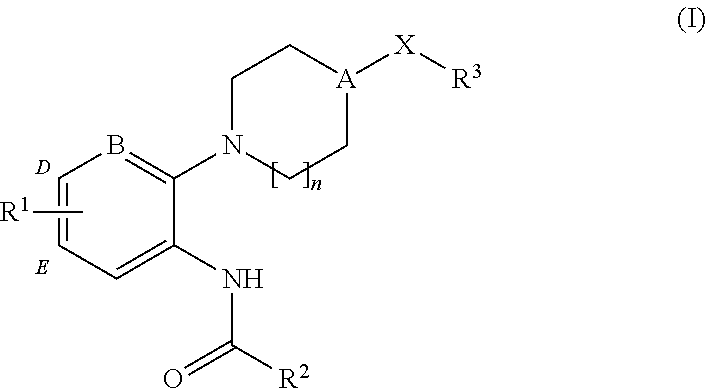
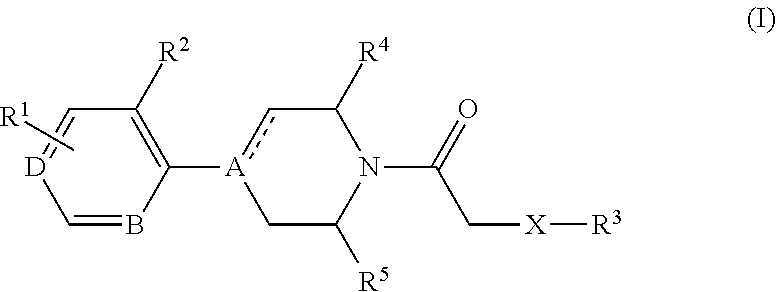
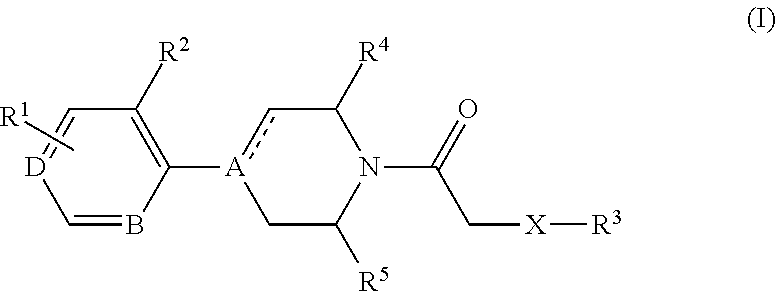
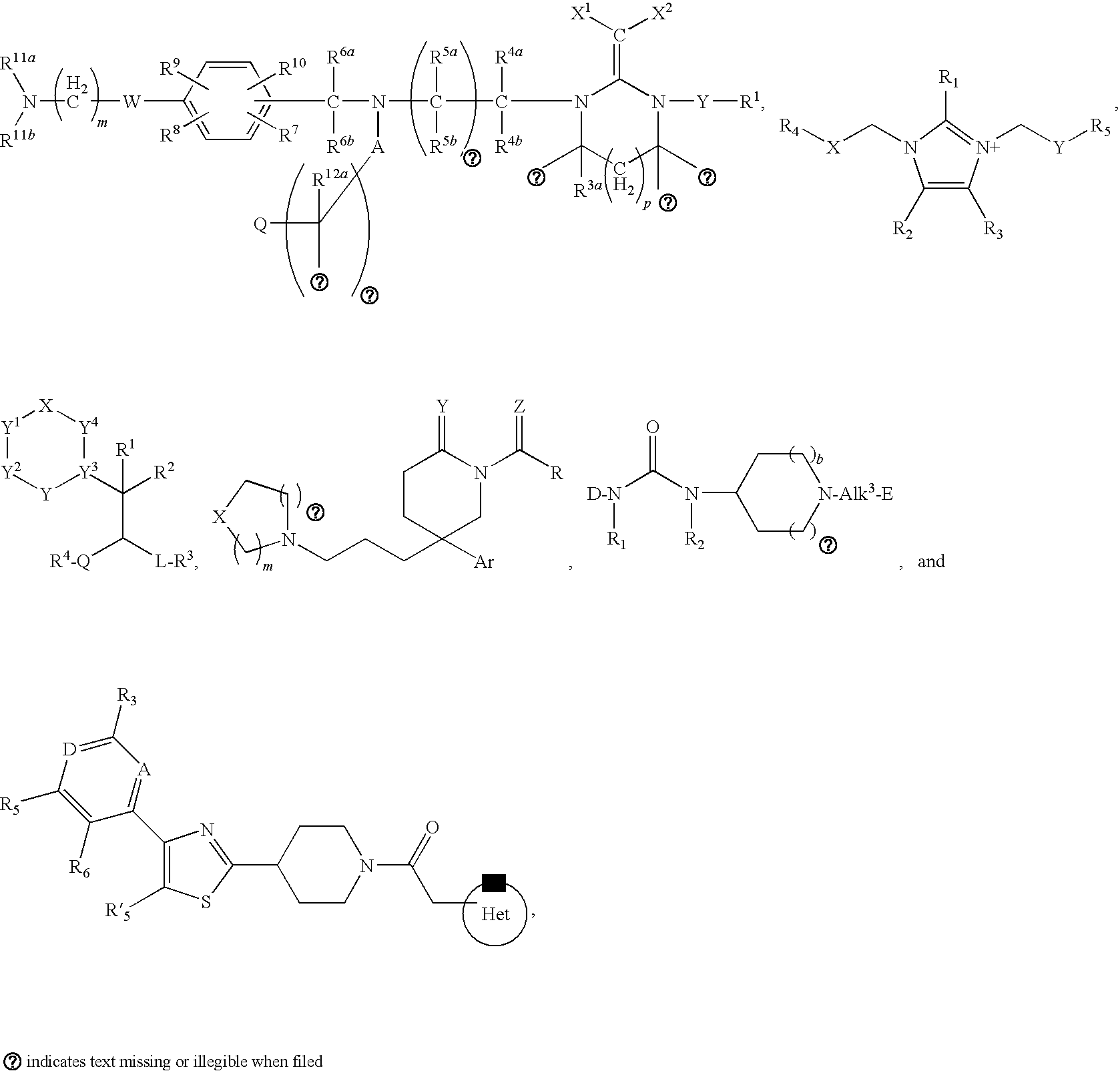
4-(benzoimidazol-2-yl)-thiazole compounds and related aza derivatives
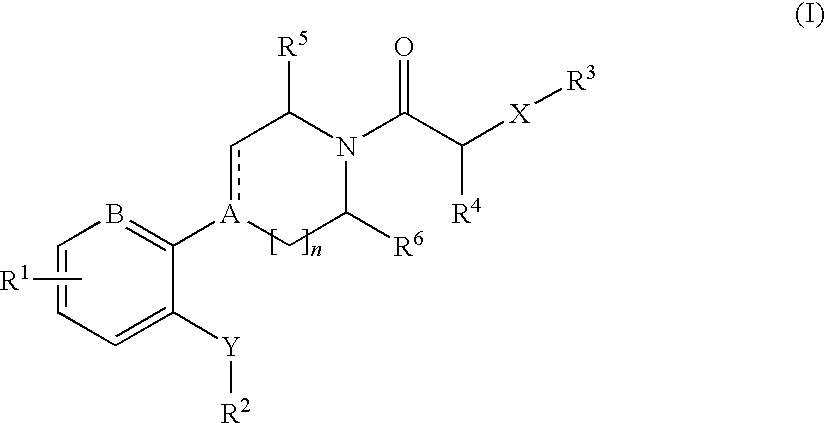
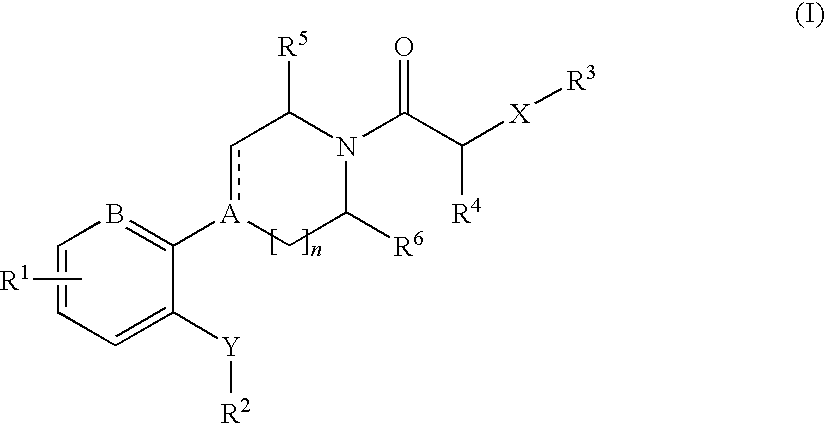
The invention relates to compounds of formula (I), wherein ring A, X, (R1)n, R2, R3, R4, R4', R5, n, and p are as described in the description. The invention also relates to pharmaceutically acceptable salts thereof, and to the use of the compounds as medicaments, especially as modulators of the CXCR3 receptor.
Owner:IDORSIA PHARM LTD
Diagnostics and remedies for interstitial pneumonia
InactiveUS20050095242A1Promote cloningHigh expressionAntipyreticAnalgesicsDiagnostic agentBULK ACTIVE INGREDIENT
A preventive agent, a diagnostic agent or a therapeutic agent for interstitial pneumonia, which comprises an anti-CCR4 antibody and / or an anti-CXCR3 antibody as an active ingredient, a diagnostic agent for discriminating between usual idiopathic interstitial pneumonia and non-specific idiopathic interstitial pneumonia which comprises an anti-CCR4 antibody and an anti-CXCR3 antibody as an active ingredient, and a method for discriminating between usual idiopathic interstitial pneumonia and non-specific idiopathic interstitial pneumonia, which comprises detecting and / or determining a Th2 cell and a Th1 cell in a sample by using an anti-CCR4 antibody and an anti-CXCR3 antibody.
Owner:KYOWA HAKKO KOGYO CO LTD
Composition and method for inducing protective vaccine response
InactiveUS20070160571A1Stimulate immune responseReduce degradationChemokinesPeptide/protein ingredientsCXCL10Vaccine response
A method for inducing a protective immune response is disclosed, the method utilizing a composition comprising a CXCR3-binding chemokine such as CXCL10 (IP-10) administered in conjunction with a protein, peptide, polynucleotide, or other target antigen.
Owner:KRATHWOHL MITCHELL
CXCR3 receptor antagonists
Owner:BOEHRINGER INGELHEIM INT GMBH
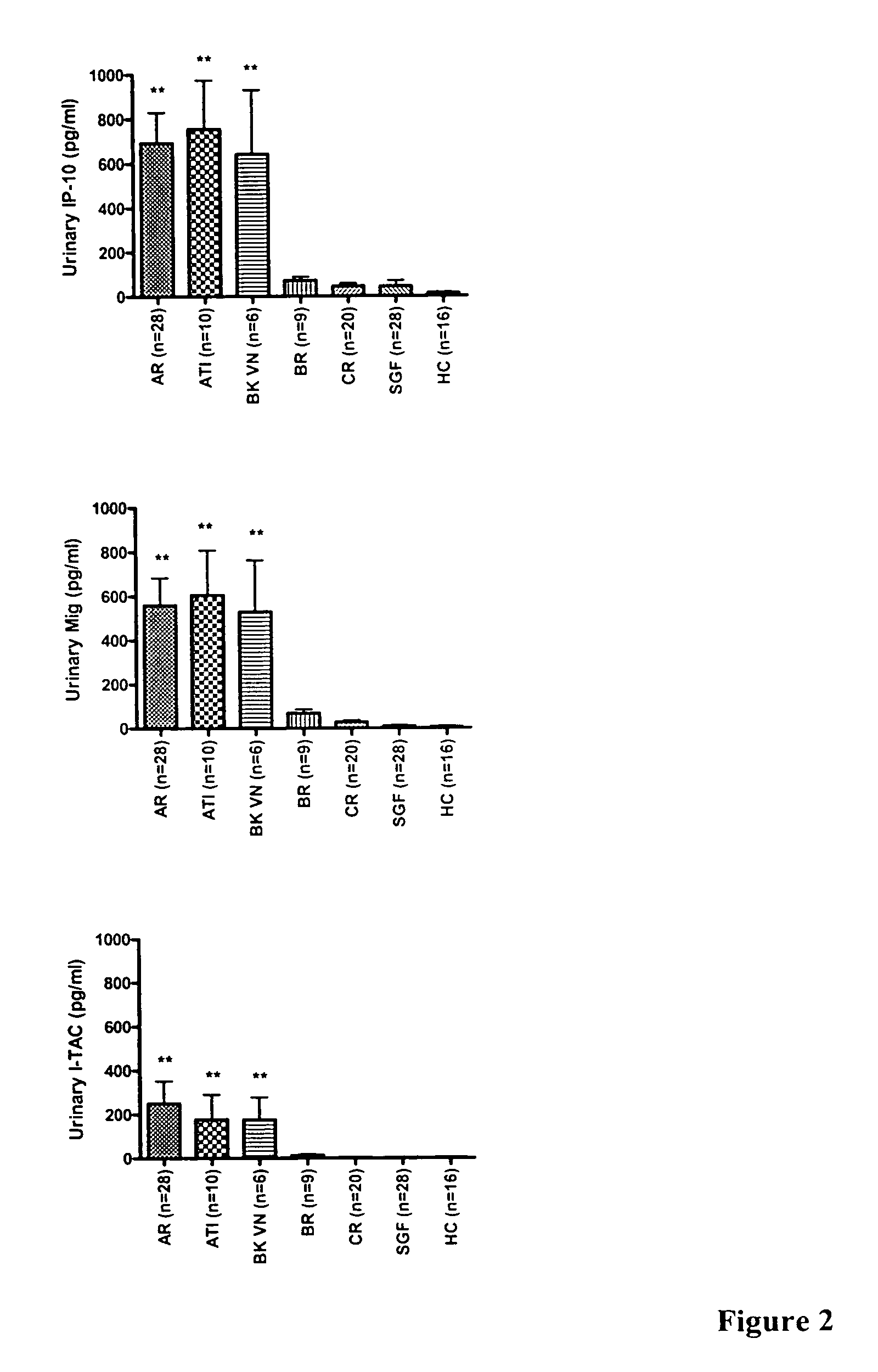
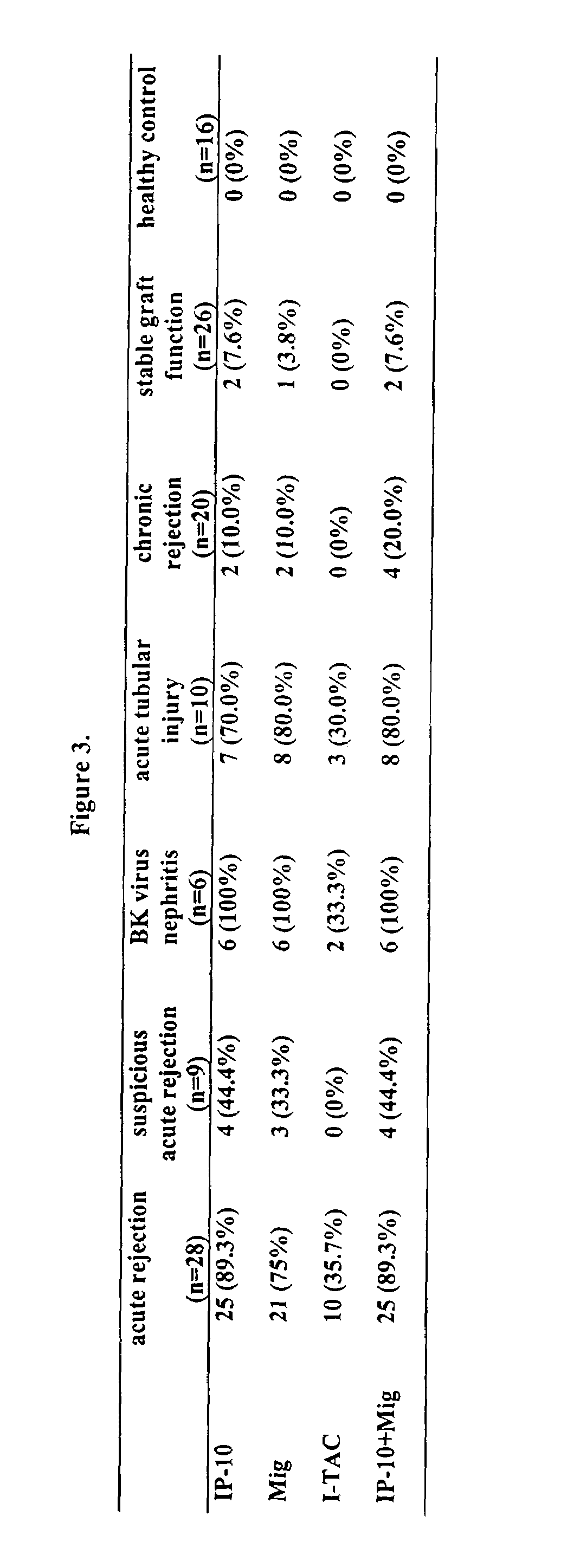
Systems and methods for identifying organ transplant risk
InactiveUS7244555B2Reduce the amount requiredMicrobiological testing/measurementDisease diagnosisOrgan transplant rejectionImproved method
The present invention relates to methods of diagnosing and predicting organ transplant rejection. In particular, the present invention relates to the detection and prediction of kidney transplant rejection by detection of CXCR3 and CCL chemokines in urine. The present invention provides improved methods of diagnosing organ rejection and determining the efficacy of anti-rejection drugs.
Owner:RENOVAR
Method for identifying ligands, inhibitors or promoters of CXC chemokine receptor 3
InactiveUS7029862B1Inhibits receptor activityStimulating receptor functionPeptide/protein ingredientsTissue cultureNatural Killer Cell Inhibitory ReceptorsT lymphocyte
The invention relates to methods of identifying ligands, and inhibitors (e.g., antagonists) or promoters (e.g., agonists) of receptor function, including methods in which host cells comprising a nucleic acid encoding a CXCR3 or variant thereof are used in an assay to identify and assess the efficacy of ligands, inhibitors or promoters. Inhibitors and promoters of receptor function can be used to modulate receptor activity, permitting selective inhibition of lymphocyte function, particularly of effector cells such as activated T lymphocytes and NK cells for therapeutic purposes.
Owner:THEODOR KOCHER INST
CXCR3 receptor antagonists
Owner:BOEHRINGER INGELHEIM INT GMBH
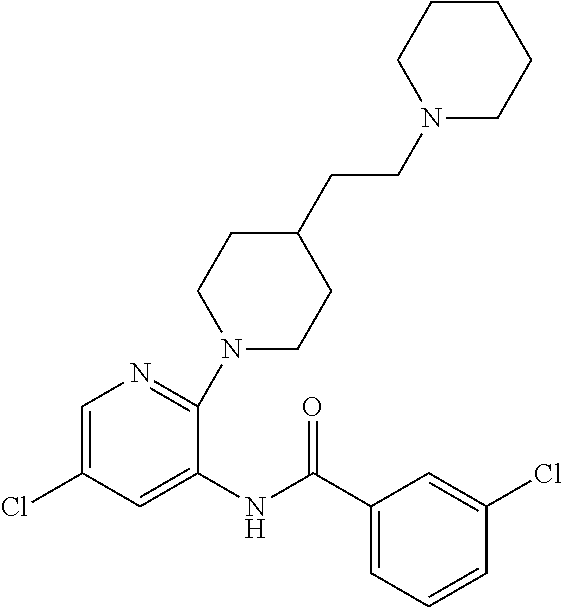
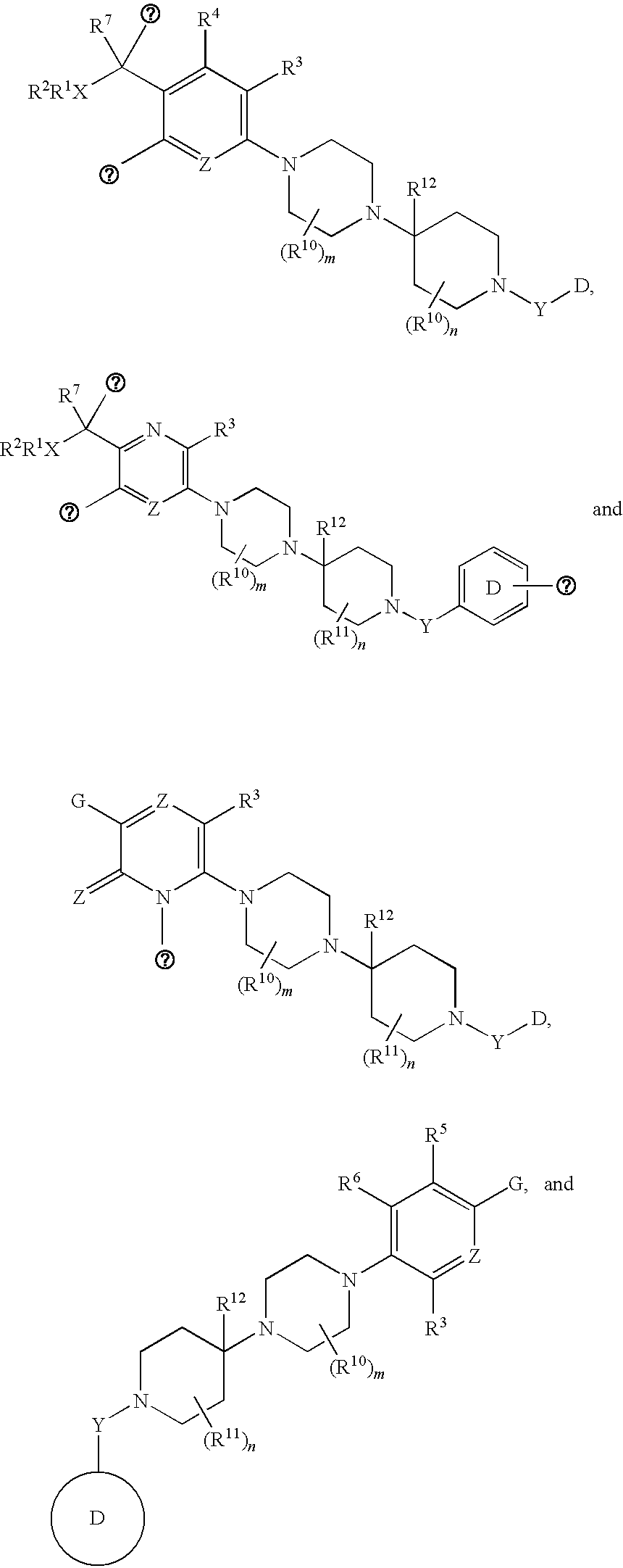
Novel heterocyclic substituted pyridine or phenyl compounds with CXCR3 antagonist activity
The present application discloses a compound, or enantiomers, stereoisomers, rotamers, tautomers, racemates or prodrug of said compound, or pharmaceutically acceptable salts, solvates or esters of said compound, or of said prodrug, said compound having the general structure shown in Formula 1 or a pharmaceutically acceptable salt, solvate or ester thereof. Also disclosed is a method of treating chemokine mediated diseases, such as, palliative therapy, curative therapy, prophylactic therapy of certain diseases and conditions such as inflammatory diseases (non-limiting example(s) include, psoriasis), autoimmune diseases (non-limiting example(s) include, rheumatoid arthritis, multiple sclerosis), graft rejection (non-limiting example(s) include, allograft rejection, zenograft rejection), infectious diseases (e.g , tuberculoid leprosy), fixed drug eruptions, cutaneous delayed-type hypersensitivity responses, ophthalmic inflammation, type I diabetes, viral meningitis and tumors using a compound of Formula 1.
Owner:SCHERING AG +1
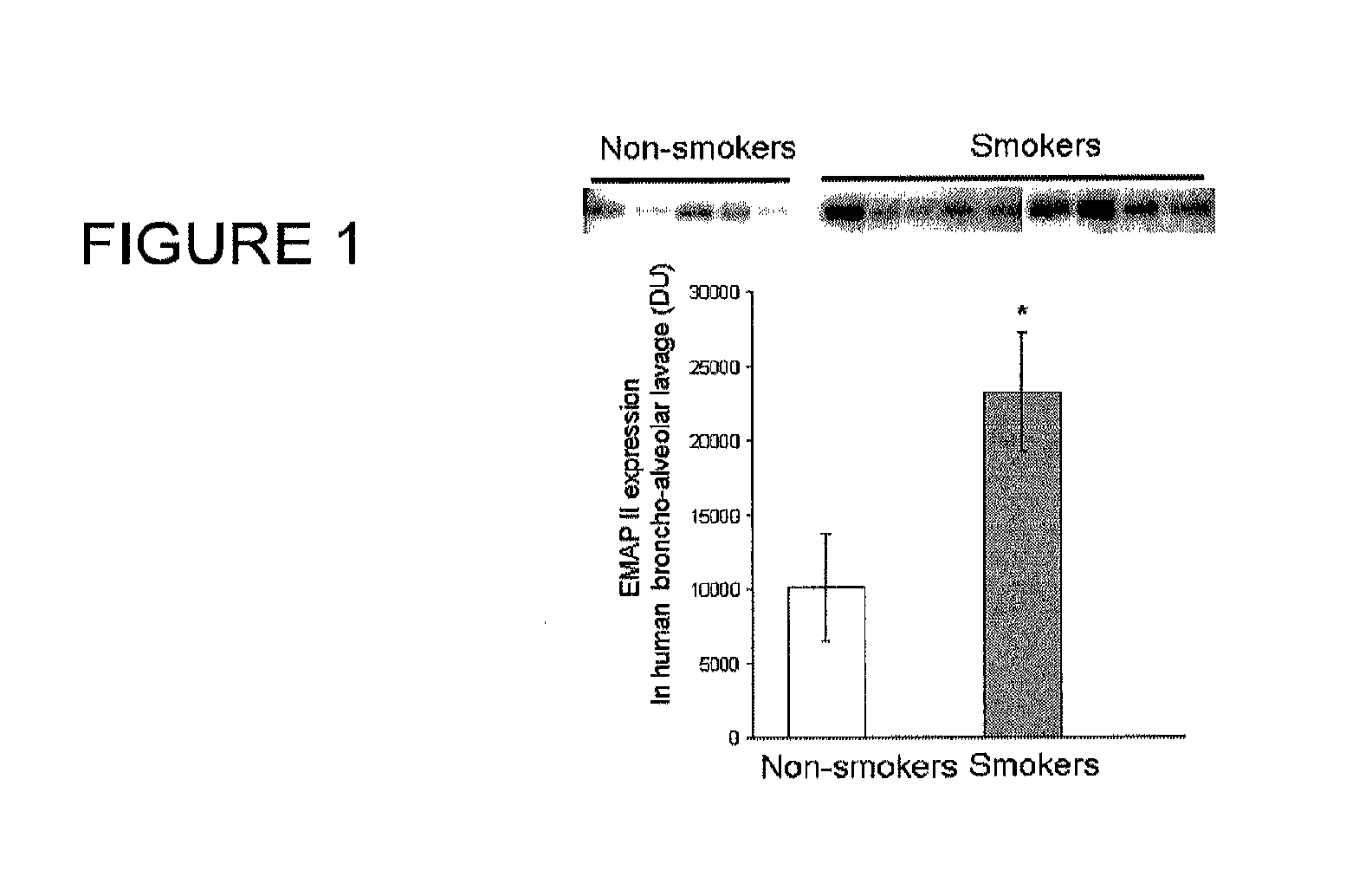
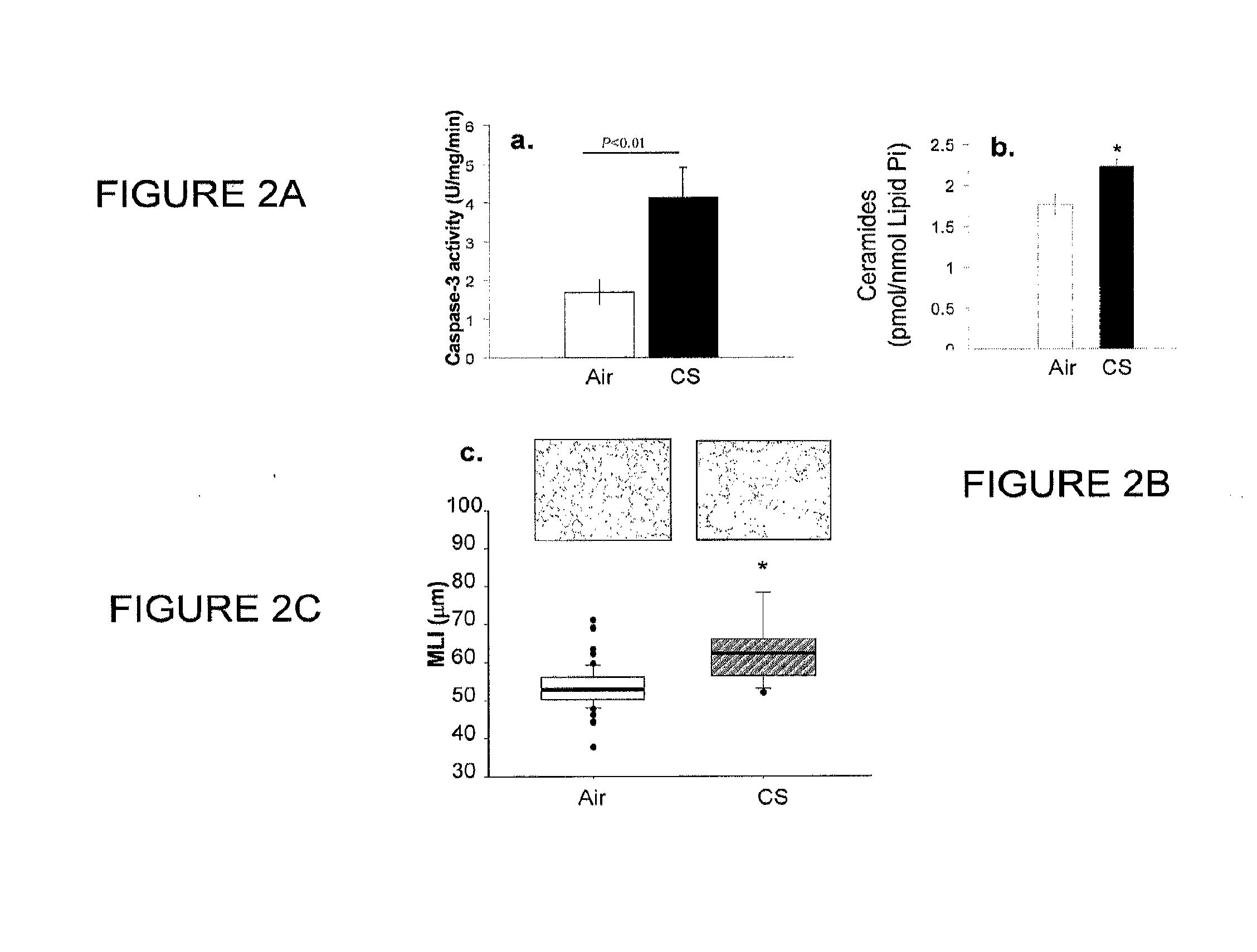
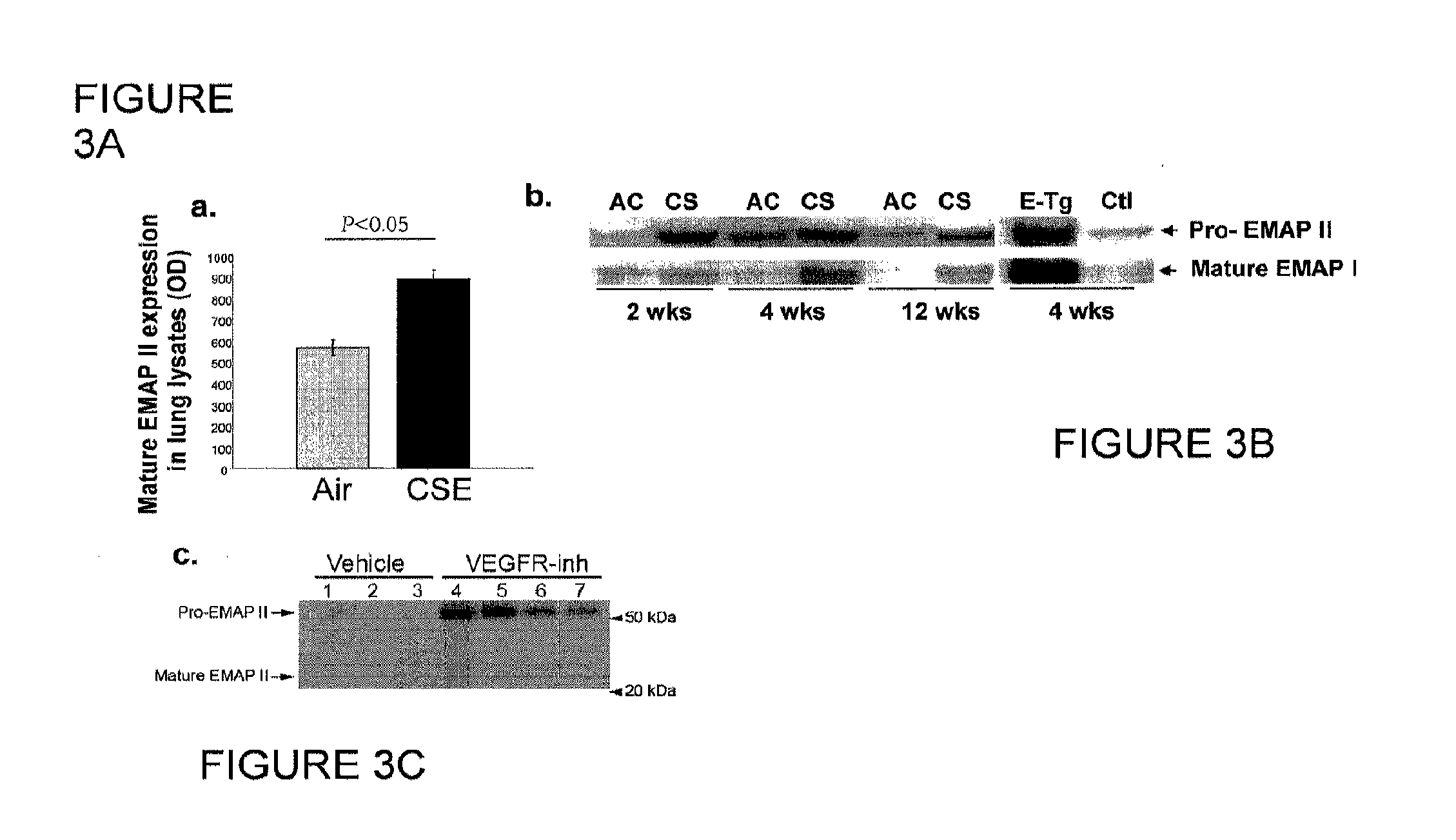
Method for diagnosing and treating emphysema
Owner:INDIANA UNIV RES & TECH CORP
Composition and Method for Inducing Protective Vaccine Response Using SDF-1
InactiveUS20080014214A1Stimulate immune responseReduce degradationViral antigen ingredientsVirus peptidesRegulatory T cellStromal cell
A method for inducing a protective immune response is disclosed, the method utilizing a composition comprising a CXCR3-binding chemokine and stromal cell-derived factor 1 which may be administered in conjunction with a protein, peptide, polynucleotide, or other target antigen to promote the development of regulatory T cells and boost the immune response to an infectious agent from which the protein, peptide, polynucleotide, or other target antigen is derived.
Owner:KRATHWOHL MITCHELL
INHIBITORS OF THE CHEMOKINE RECEPTOR CxCR3
This invention is directed to a 3-(amido or sulphamido)-4-(4-substituted-azinyl)benzamide or benzsulphonamide compound as defined herein. The 3-(amido or sulphamido)-(4-substituted-azinyl)benzamide or benzsulphonamide compound is useful as a inhibitor of the chemokine receptor CxCR3, and for preventing or treating a CxCR3 chemokine receptor mediated disease or condition related thereto in a patient in need of such.
Owner:SANOFI SA
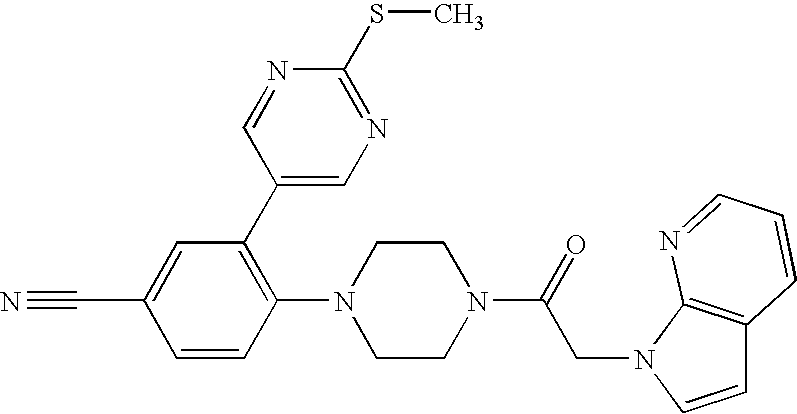
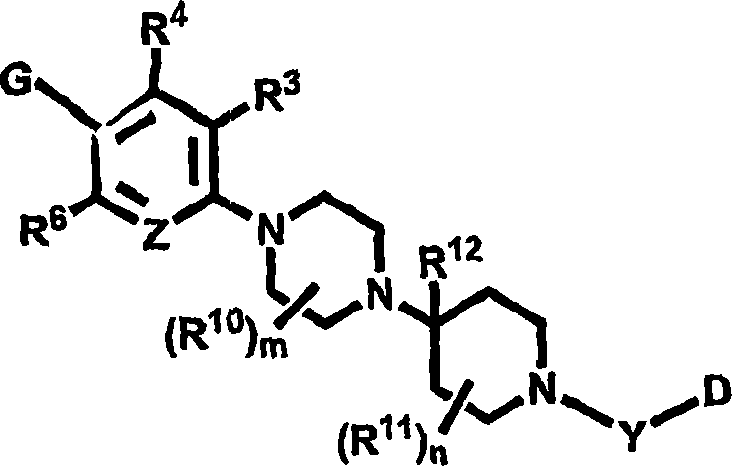
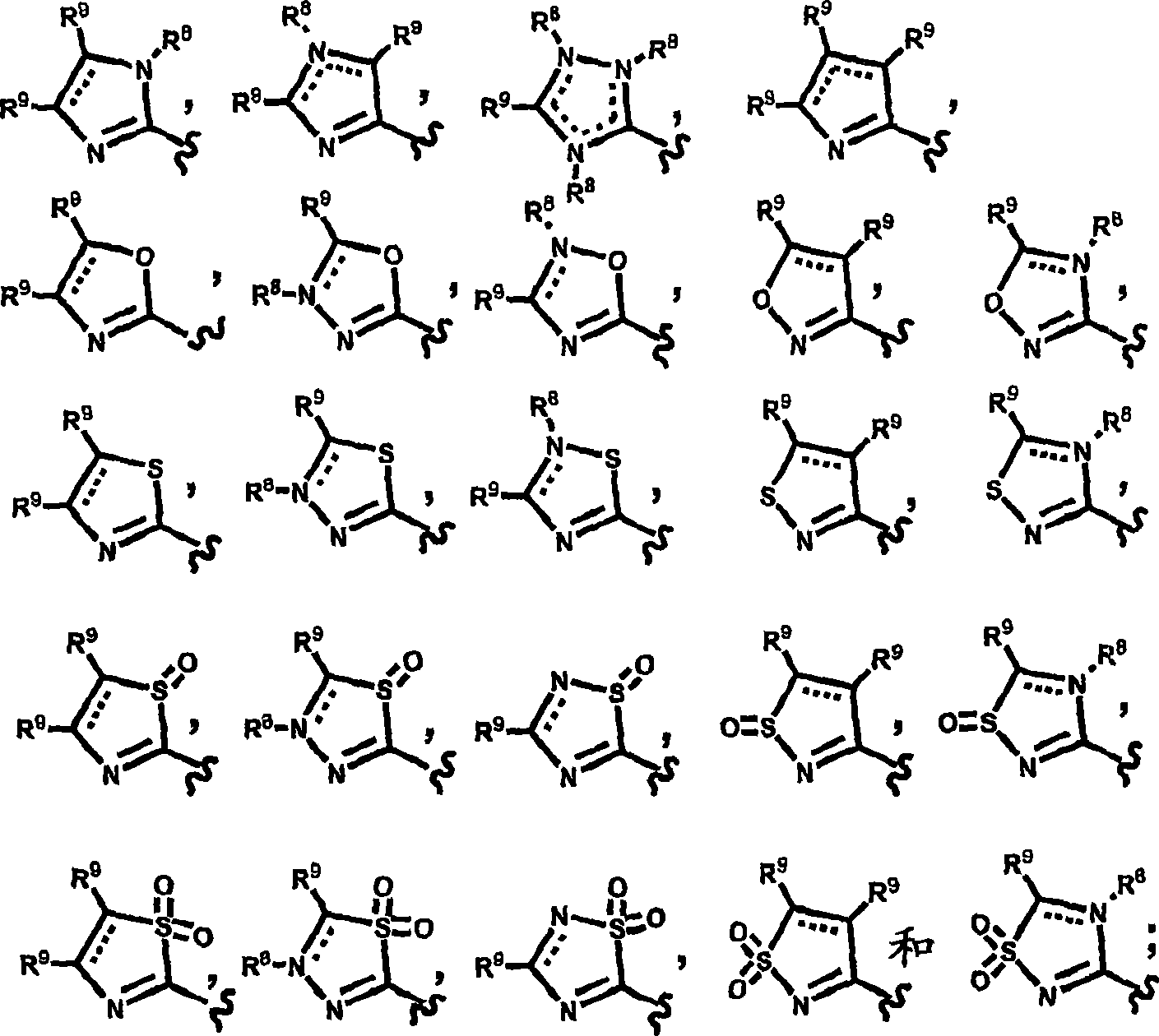
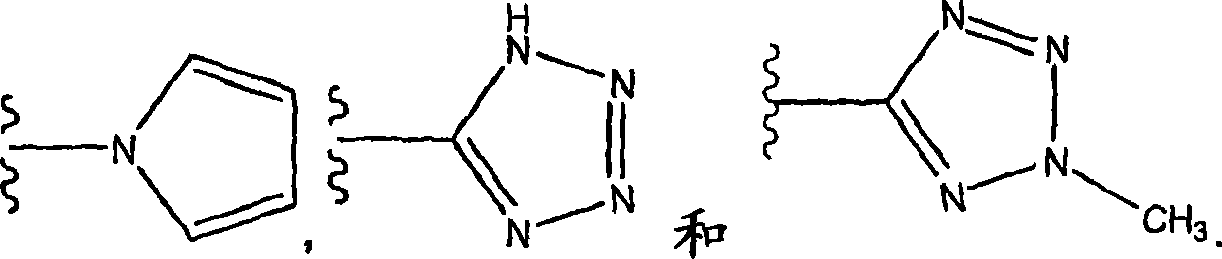
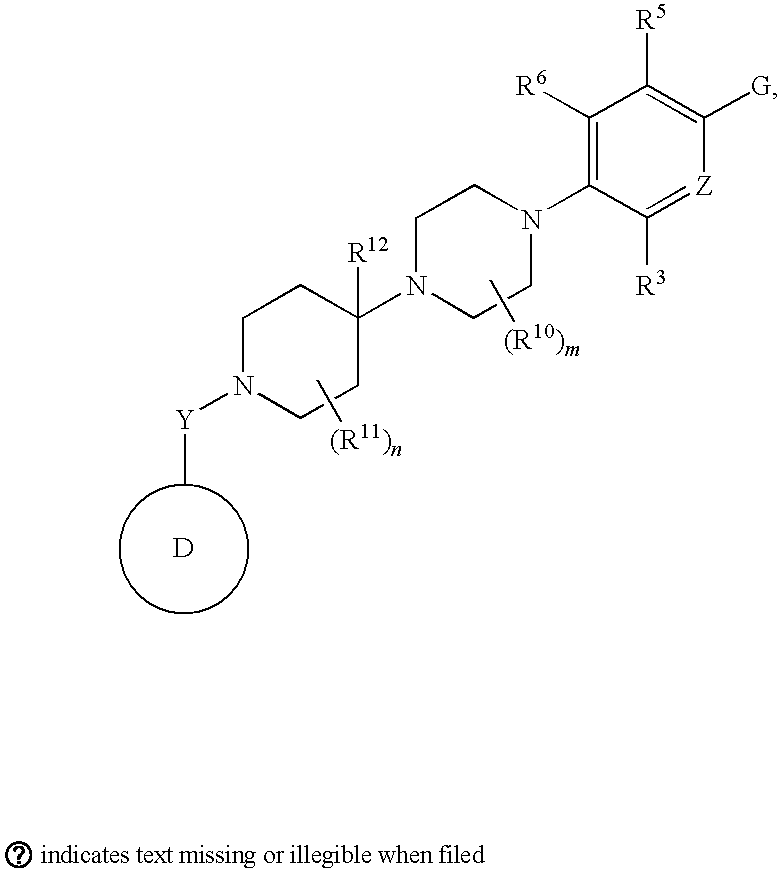
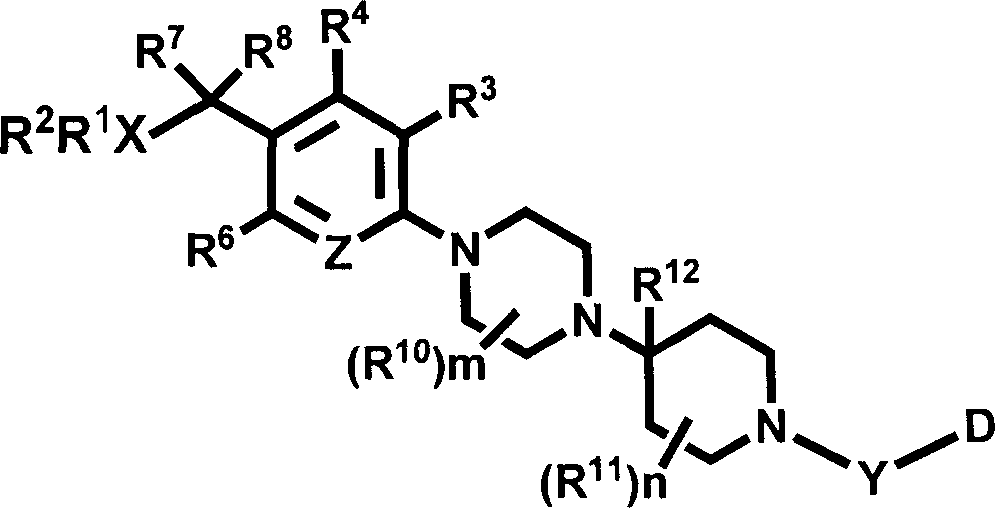
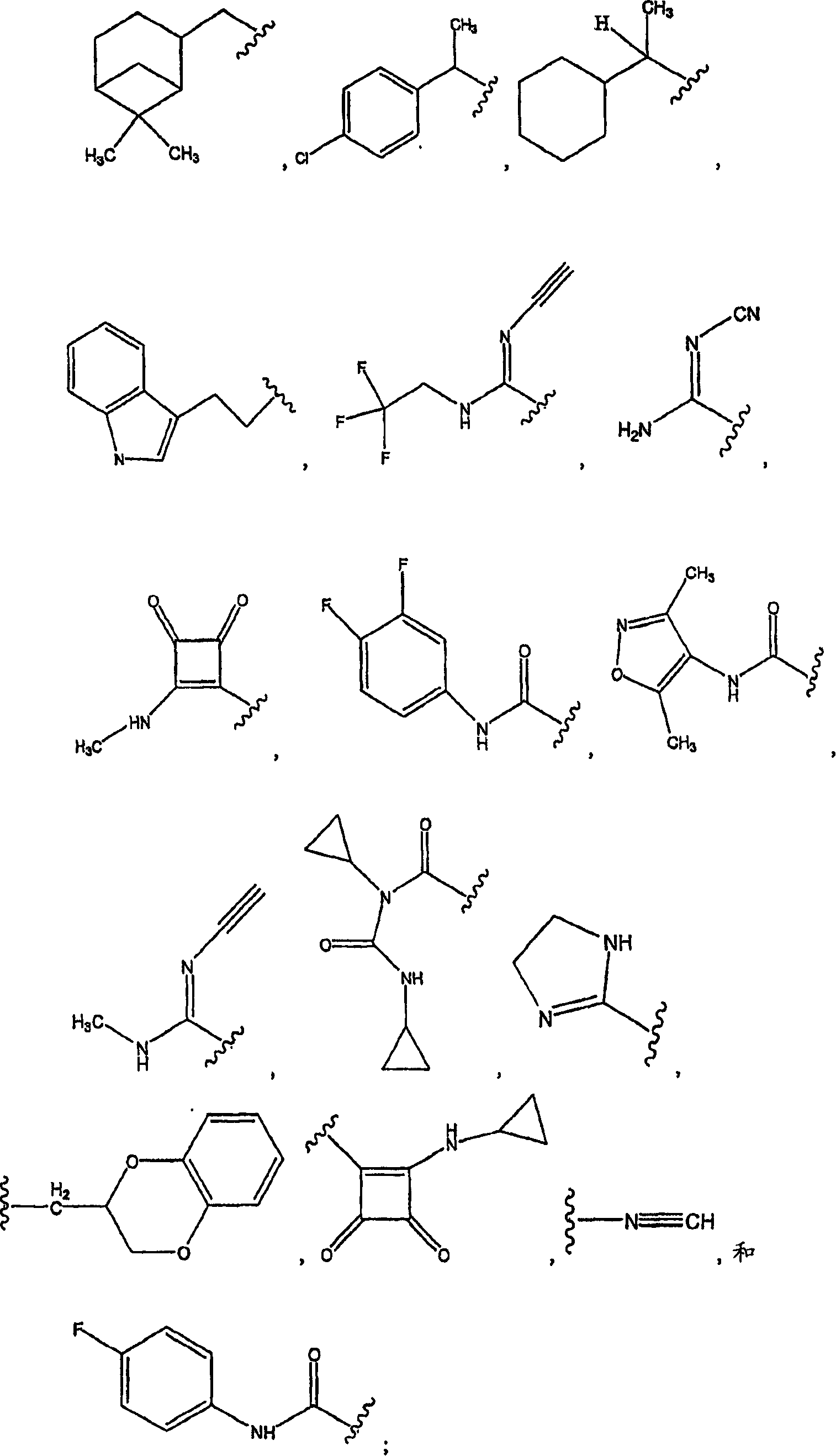
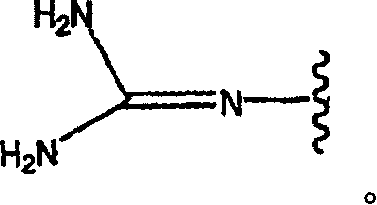
Pyridyl and phenyl substituted piperazine-piperidines with CXCR3 antagonist activity
The present application discloses a compound, or enantiomers, stereoisomers, rotamers, tautomers, racemates or prodrug of said compound, or pharmaceutically acceptable salts, solvates or esters of said compound, or of said prodrug, said compound having the general structure shown in Formula 1: and the pharmaceutically acceptable salts, solvates and esters thereof. Also disclosed is a method of treating chemokine mediated diseases, such as, palliative therapy, curative therapy, prophylactic therapy of certain diseases and conditions such as inflammatory diseases (non limiting example(s) include, psoriasis), autoimmune diseases (non limiting example(s) include, rheumatoid arthritis, multiple sclerosis), graft rejection (non limiting example(s) include, allograft rejection, xenograft rejection), infectious diseases (e.g , tuberculoid leprosy), fixed drug eruptions, cutaneous delayed-type hypersensitivity responses, ophthalmic inflammation, type I diabetes, viral meningitis and tumors using a compound of Formula 1.
Owner:SCHERING AG +1
Pharmaceutical composition comprising cxcr3 inhibitor
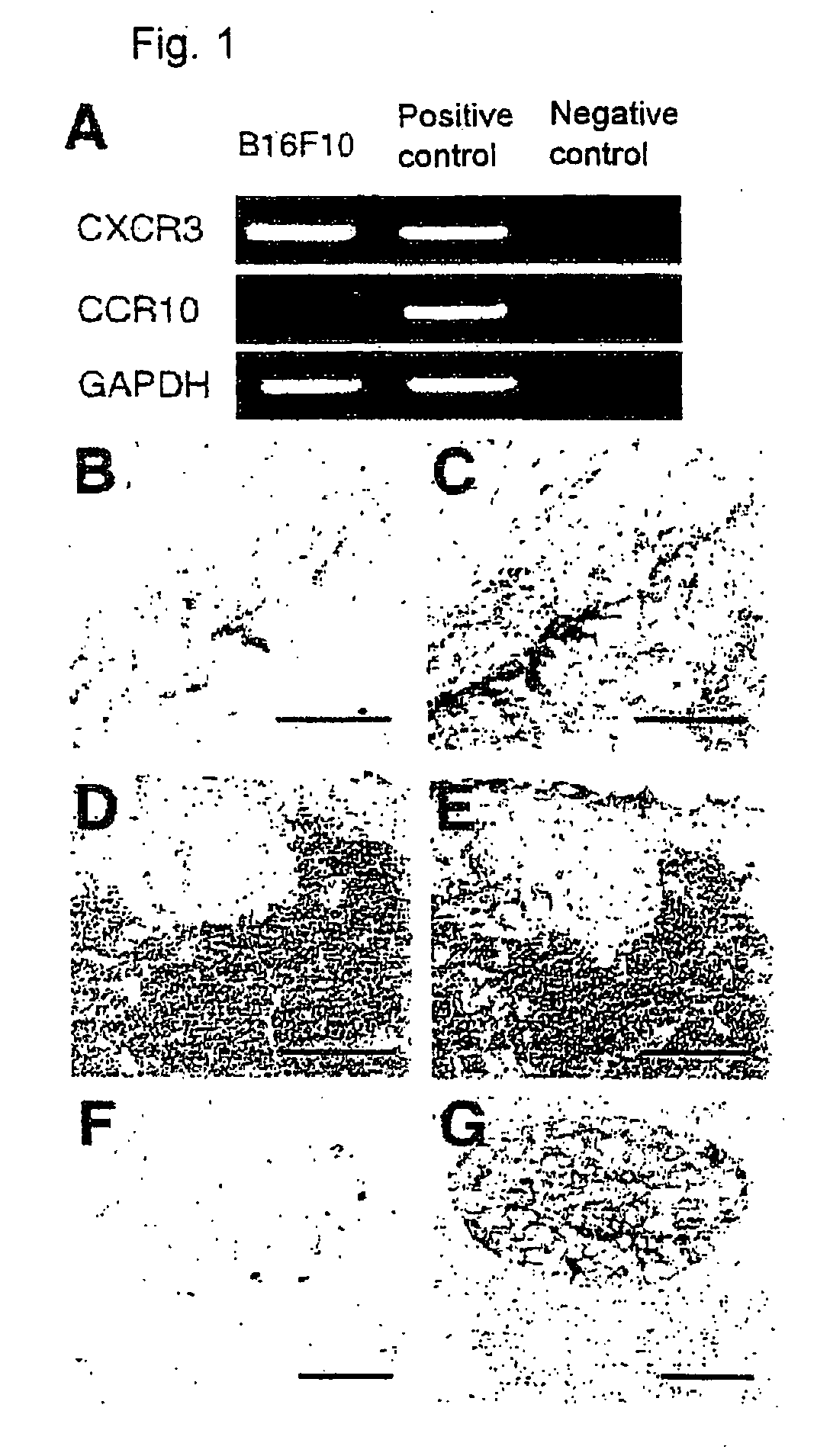
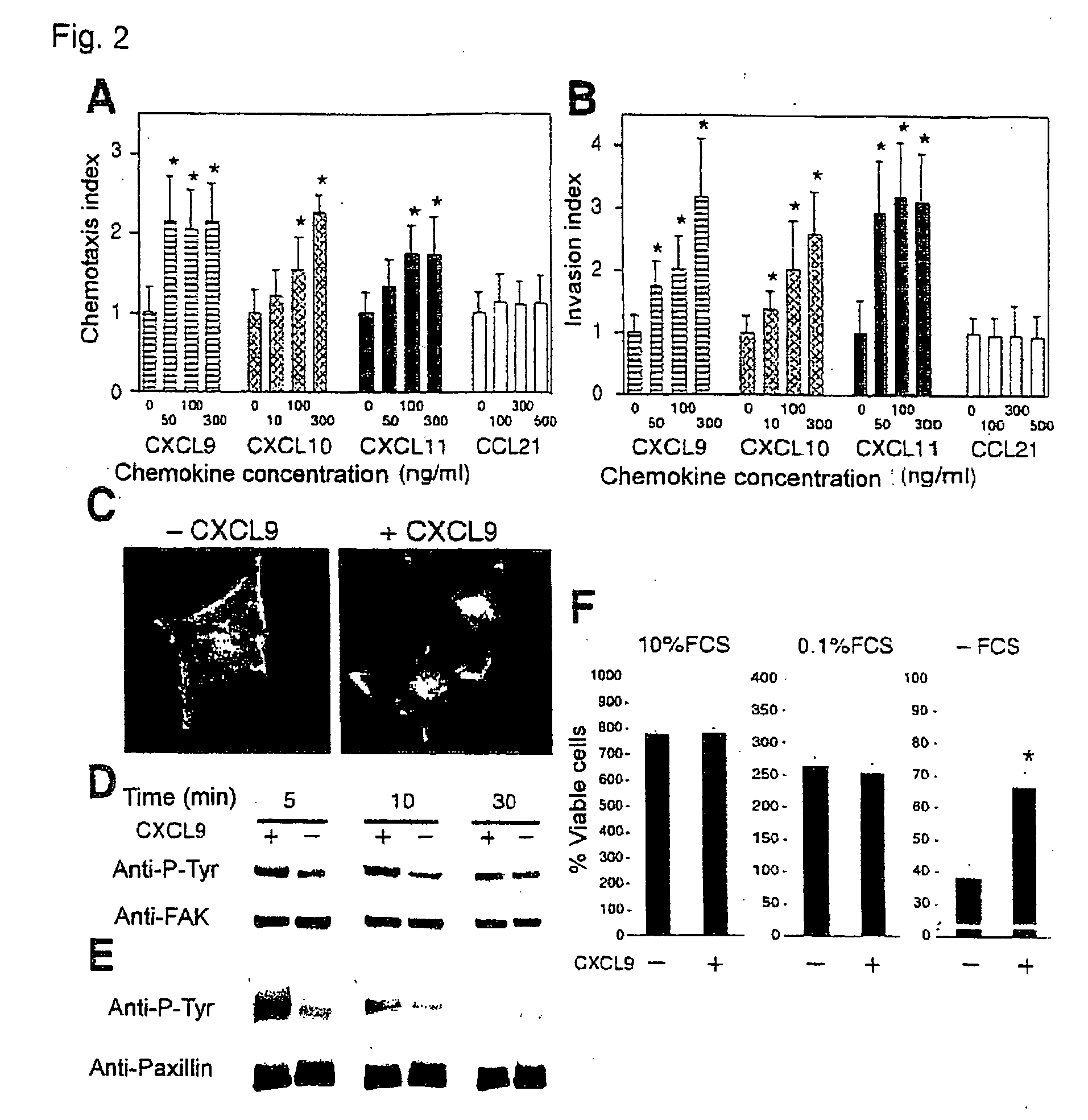
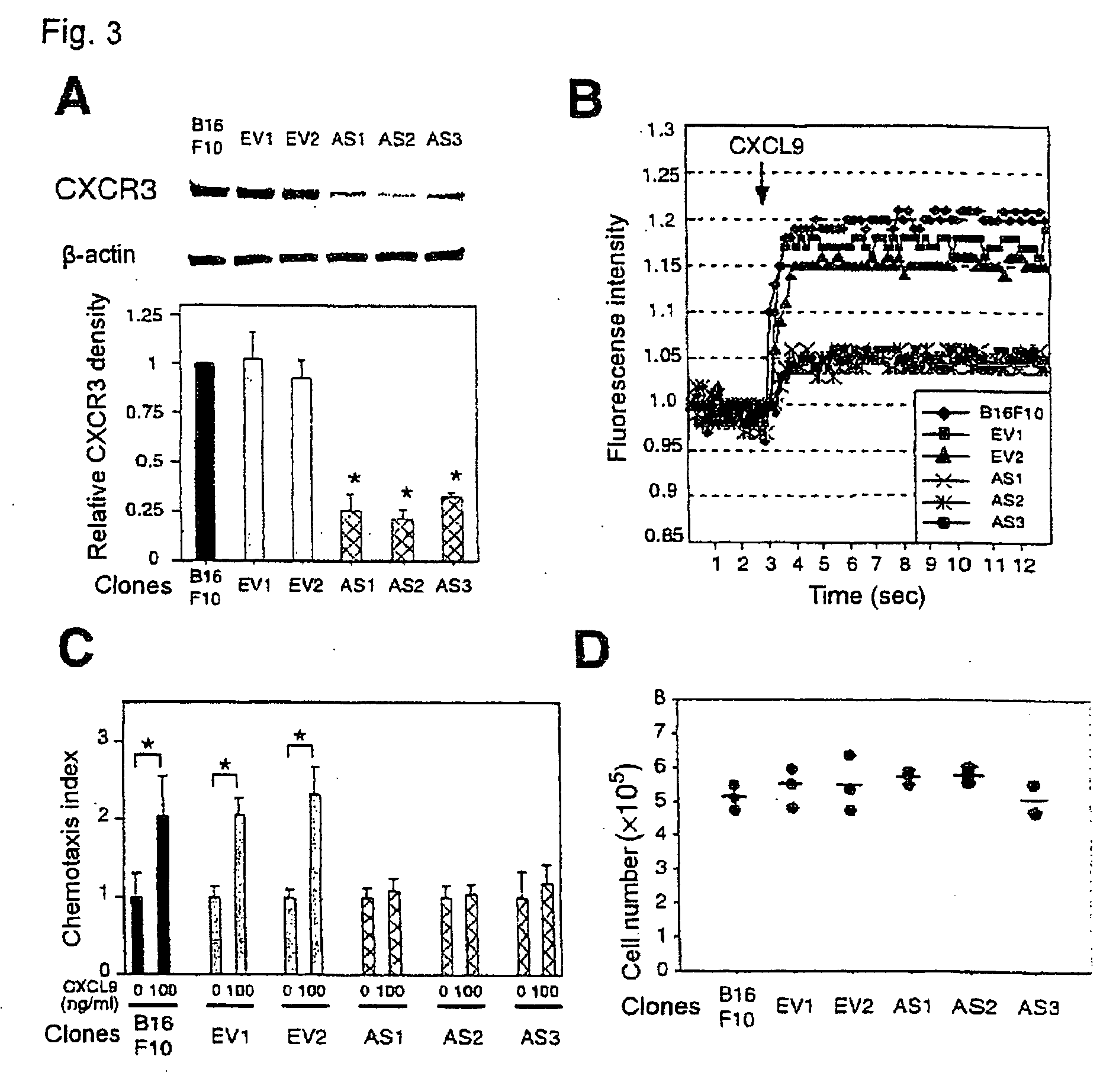
InactiveUS20090208486A1Organic active ingredientsGenetic material ingredientsPharmaceutical drugOncology
Owner:KYOTO UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com