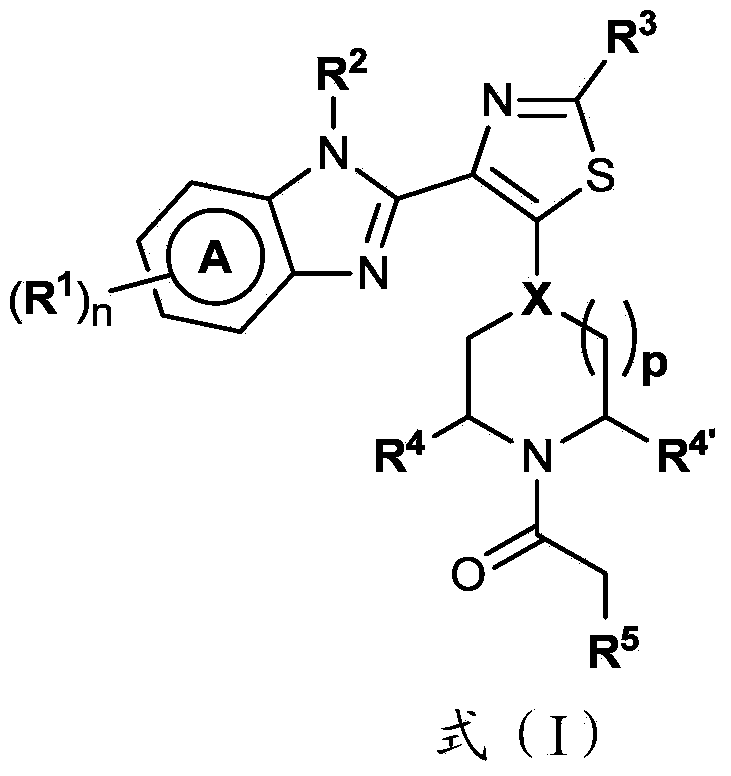
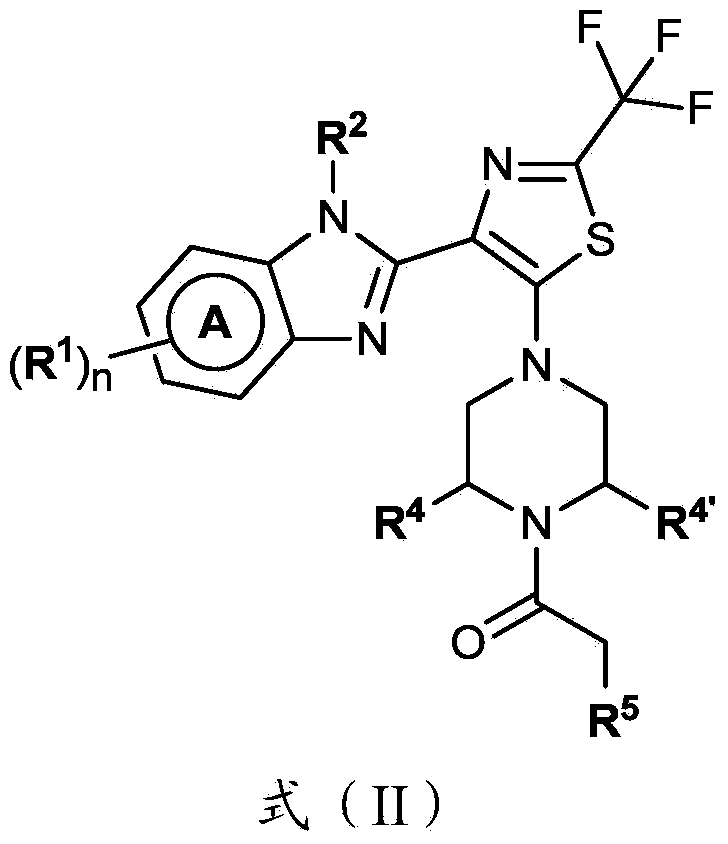
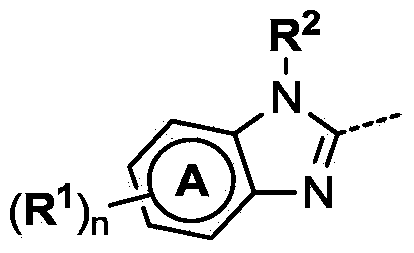
4-(benzoimidazol-2-yl)-thiazole compounds and related aza derivatives
A compound, phenyl technology, applied in the field of CXCR3 receptor modulators, can solve the problem of low expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0848] Example 1: 2-benzimidazol-1-yl-1-{4-[4-(1H-benzimidazol-2-yl)-thiazol-5-yl]-piperazin-1-yl}-B ketone:
[0849] 1.1 tert-butyl 4-(4-methoxycarbonyl-thiazol-5-yl)-piperazine-1-carboxylate:
[0850] To a solution of methyl 5-bromo-1,3-thiazole-4-carboxylate (10 g) in MeCN (120 mL) was added 1-Boc-piperazine (8.56 g) followed by DBU (10.1 mL). The resulting solution was stirred at 80 °C for 5 h. The reaction mixture was diluted with EA and water. The layers were separated and the organic phase was further washed with water. The combined aqueous layers were extracted with EA. by Na 2 SO 4 The combined organic layers were dried, filtered and evaporated in vacuo. The crude product was purified by CC (Biotage, SNAP 100 g cartridge, solvent A: Hept; solvent B: EA; gradient of %B: 2CV10%, 10 to 50% in 12CV, 3CV50%) to give 7.65g yellow oil. LC-MS(B):t R =0.79min; [M+H] + :328.37.
[0851]1.2.4-(4-Carboxyl-thiazol-5-yl)-piperazine-1-carboxylic acid tert-butyl ester:
...
Embodiment 8
[0861] Example 8: 1-{4-[4-(1H-benzimidazol-2-yl)-thiazol-5-yl]-piperazin-1-yl}-2-(6-chloro-imidazo[1 , 2-b] pyridazin-2-yl)-ethanone:
[0862] To 6-chloroimidazo[1,2-b]pyridazin-2-yl)acetic acid (12.7 mg) was added Intermediate 1.4 (20.8 mg) in DMF / DIPEA (0.5 mL, 5 / 1 ) and HOAT ( 8.17 mg) in DMF (0.5 mL), then Si-DCC (0.96 mmol / g, 180 mg) was added. The reaction mixture was stirred at 40 °C for 24 h. Add PL-HCO 3 (2.06mmol / g, 120mg) and PL-DETA (7.99mmol / g, 23mg) and the reaction mixture was stirred for another 3h. The resin was filtered, washed five times with 1 mL DCM / MeOH 1:1, and the resulting solution was evaporated in vacuo. The residue was dissolved in DMSO / MeCN 1:4 (0.5 mL) and purified by preparative LC-MS (II) to give 14 mg of a white solid. LC-MS(A):t R =0.57min; [M+H] + :479.2.
[0863] Examples 9 to 13 were synthesized according to the procedure described in Example 8, starting from the appropriate acid derivative. LC-MS data for Examples 9 to 13 are list...
Embodiment 14
[0865] Example 14: 1-{4-[4-(1H-benzimidazol-2-yl)-thiazol-5-yl]-piperazin-1-yl}-2-imidazo[4,5-b] Pyridin-3-yl-ethanone:
[0866] 14.1. Imidazo[4,5-b]pyridin-3-yl-benzyl acetate:
[0867] To a brown solution of 4-azabenzimidazole (4.75 g) in DMF (80 mL) was added benzyl bromoacetate (6.58 mL) followed by Cs 2 CO 3 (25.9g). The resulting light brown suspension was stirred overnight. The reaction mixture was diluted with EA and washed with water and saturated NH 4 Cl aqueous solution was washed twice. The aqueous layer was extracted twice with EA. by MgSO 4 The combined organic layers were dried, filtered and evaporated under reduced pressure. By CC (Biotage, SNAP 100g cartridge, Solvent A:DCM; Solvent B:DCM / MeOH8:2; Gradient %B: 0 to 5% in 3CV, 5% in 5CV, 5 to 15% in 5CV, 3CV 15% B) Purification of the crude product gave 4.99 g of the desired compound as a yellow solid. LC-MS(C):t R =0.59min; [M+H] + :267.86.
[0868] 14.2. Imidazo[4,5-b]pyridin-3-yl-acetic acid:
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com