Application of iodine-containing chrysin derivative in preparation of medicine with radiation-proof effect
A technique for chrysin and derivatives is applied in the application field of preparing anti-radiation damage medicines, which can solve the problems of poor effect, large toxic and side effects, and poor protection effect, and achieves small toxic and side effects, no weight loss, and no Effects that cause systemic toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
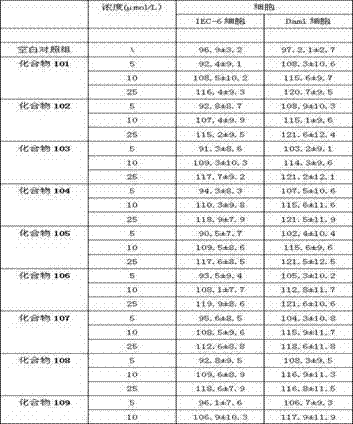
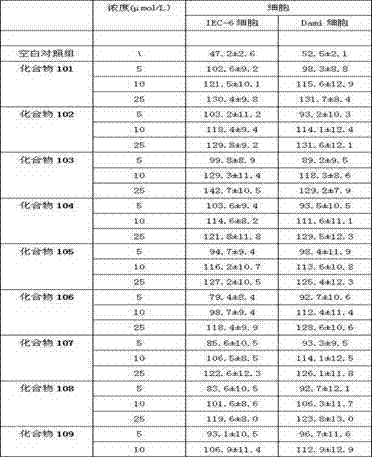
Examples
Embodiment 1
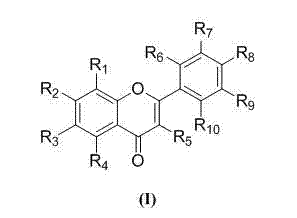
[0041] Embodiment 1 4'-iodochrysin (compound 101 ) preparation
[0042] (1) React 0.1mol phloroglucinol with 0.2mol chloroacetonitrile in 50mL ether, use zinc chloride as a catalyst, pass dry HCl gas for 2 hours under the condition of ice-salt bath, put it in the refrigerator for one day, and pass HCl again Gas 2h, placed in the refrigerator for three days. Pour off ether, wash with anhydrous ether (20mL×3), transfer the crude extract to a round bottom flask with hot water, reflux for 1h, let stand overnight, and recrystallize with water to obtain 2,4,6-trihydroxy- α-Chloroacetophenone.
[0043] (2) Mix 1.0mmol 2,4,6-trihydroxy-α-chloroacetophenone, 1.4mmol 4-iodobenzaldehyde and 0.5mL 95% ethanol, shake well, slowly add 2mL 10% NaOH dropwise, then vigorously Shake for a while, place at room temperature for 3 days, acidify with dilute hydrochloric acid, then place at room temperature for 2-3 days, filter with suction, and recrystallize the crude product with acetone or 95% ...
Embodiment 2
[0045] Embodiment 2 3'-iodochrysin (compound 102) and 2′-iodochrysin (compound 103 ) preparation: carry out with reference to Example 1. The relevant data are as follows:
[0046] compound 102 : MS (EI, 70ev) m / z: 380; Anal. Calcd. For C 15 h 9 IO 4 : C, 47.59; H, 2.48; I, 33.45; O, 16.48; Found C, 47.49; H, 2.42; I, 33.38; O, 16.81.
[0047] compound 103 : MS (EI, 70ev) m / z: 380; Anal. Calcd. For C 15 h 9 IO 4 : C, 47.39; H, 2.46; I, 33.28; O, 16.97; Found C, 47.29; H, 2.39; I, 33.36; O, 17.04.
Embodiment 3
[0048] Example 3 5,7-dimethoxy-4'-iodochrysin (compound 104 ) and 7-methoxy-4′-iodochrysin (compound 108 ) preparation
[0049] 0.3mmol 4′-iodochrysin and 3.0mmol methyl bromide in 25mL acetone, using anhydrous potassium carbonate as a catalyst, heated to reflux for 12h, and used dichloromethane: acetone as 5:1 column chromatography to obtain 5,7-dimethyl Oxygen-4′-iodochrysine (compound 104 ) and 7-methoxy-4′-iodochrysin (compound 108 ), the relevant data are as follows:
[0050] compound 104 : MS (EI, 70ev) m / z: 408; Anal. Calcd. For C 17 h 13 IO 4 : C, 50.02; H, 3.21; I, 31.09; O, 15.68; Found C, 50.12; H, 3.27; I, 31.15; O, 15.46.
[0051] compound 108 : MS (EI, 70ev) m / z: 408; Anal. Calcd. For C 17 h 13 IO 4 : C, 50.23; H, 3.14; I, 31.25; O, 15.38; Found C, 50.33; H, 3.15;
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap