Chicken Newcastle Disease Virus Attenuated Strain, Inactivated Vaccine and Its Application
A technology for chicken Newcastle disease virus and chicken Newcastle disease, which is applied to attenuated strains of chicken Newcastle disease virus, inactivated vaccines and their application fields, and can solve the problems of virus hemagglutination spectrum, differences in virus thermal stability and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Isolation, cultivation and identification of chicken Newcastle disease virus attenuated strain (DL11 strain) of embodiment 1
[0045]1. Collection of disease materials
[0046] Chicken disease materials come from a farm in the suburbs of Daqing City, Heilongjiang Province. The livers of dying chickens are collected. After grinding, they are treated with 1,500 units of penicillin and streptomycin solution at 4°C for 12 hours, centrifuged at 2,000 r / min, and the supernatant is taken to inoculate chicken embryos and collected for 48 hours. 96h dead chicken embryo allantoic fluid.
[0047] 2. Hemagglutination (HA) and hemagglutination inhibition (HI) tests of virus isolates
[0048] Hemagglutination test was carried out on the harvested chicken embryo fluid, and the hemagglutination value of HA was 8log 2 .
[0049] HI test was carried out with avian influenza H5, H7 and H9 subtype standard positive sera, ND and EDS standard positive sera and chicken embryo allantoic flu...
Embodiment 2
[0073] The preparation of embodiment 2 chicken Newcastle disease virus DL11 strain seed virus
[0074] 1. Establishment of basic seed batches
[0075] Use the screened 20th generation seed virus (CGMCC NO.7958) to inoculate the CEF grown into a monolayer, and after adsorption at 37°C for 1 hour, add DMEM containing 2% calf serum, and cool to 37°C, 5% CO 2 Environmental culture, after 80% of the cells have lesions, freeze and thaw three times at -20°C, harvest the cytotoxicity, and harvest after two freeze-thaws. Continuous passage does not exceed 3 generations.
[0076] 2. Production seed batch establishment
[0077] Take the basic seeds, dilute them 10,000 times with sterilized normal saline, insert 1% of the virus culture solution into the monolayer cell culture, absorb at 37°C for 1 hour, add DMEM containing 2% calf serum, to 37°C, 5% CO 2 Environmental culture, after 80% of the cells have lesions, freeze and thaw 3 times at -20°C, and harvest the toxic cell culture med...
Embodiment 3
[0078] The preparation of embodiment 3 chicken Newcastle disease inactivated vaccines
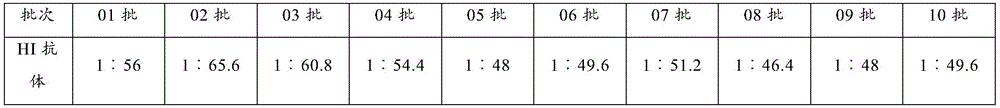
[0079] In order to verify the characteristics of cytotoxicity, 10 batches of inactivated vaccines were prepared with the same method using the screened 20th-generation seed virus (CGMCC NO.7958), and the relevant technical indicators were determined, as described below.
[0080] 1. Preparation of virus liquid
[0081] 1.1 Preparation of cells
[0082] Take SPF chicken embryos aged 9-11 days, remove the head, limbs and viscera, and cut them into 1mm 3 Tissue blocks left and right. Wash 3 times with PBS, digest with 0.25% trypsin for 5-15min, discard the trypsin, add DMEM containing 10% calf serum, blow the cells evenly with a pipette, put them into a culture bottle, and culture them at 37°C until Cells were used after growing into a monolayer.
[0083] 1.2 Inoculation and harvest
[0084] Wash the monolayered CEF with PBS for 3 times, inoculate the 20th generation seed virus (CGMCC NO.7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com