Body cavity effusion suppressant
An inhibitor, pleural effusion technology, applied in the direction of anti-inflammatory agents, blood diseases, extracellular fluid diseases, etc., can solve the problem of not getting it, and achieve the effect of reducing incorrect puncture and chest pain.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
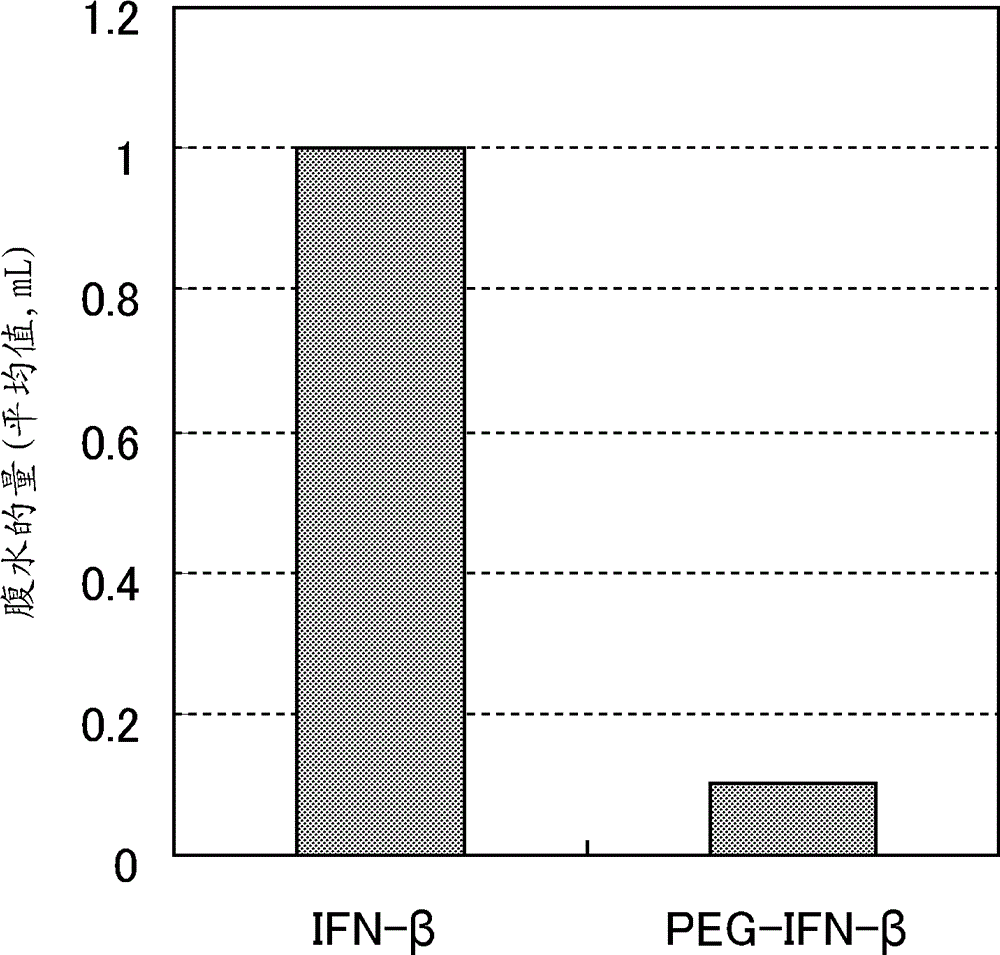
[0085] [Example 1] Preparation of covalent conjugates of interferon-β and PEG
[0086] According to the method described in Example 6 of Japanese Patent No. 4,850,514, a "covalent conjugate of interferon-β and PEG" was prepared, wherein a PEG molecule with a molecular weight of 42,000 was mixed with recombinant human interferon-β (SEQ ID NO : 1) The amino group of lysine at position 134 of the amino acid sequence is covalently bonded. Specifically, ethylene glycol (final concentration: 20%) was added to recombinant human interferon-β (final concentration: 200 μg / ml), and adjust the pH to 7.6 with 1 M disodium hydrogen phosphate solution. Hydroxysuccinimide ester-activated PEG (molecular weight: 42,000; product number 61G99122B01; NOF Corporation) was added thereto and mixed, and a binding reaction was performed overnight at 4°C. To this binding reaction solution was added 10 mM acetate buffer (pH 4.5) in an amount greater than 5 times its volume, and the resultant was appli...
Embodiment 2
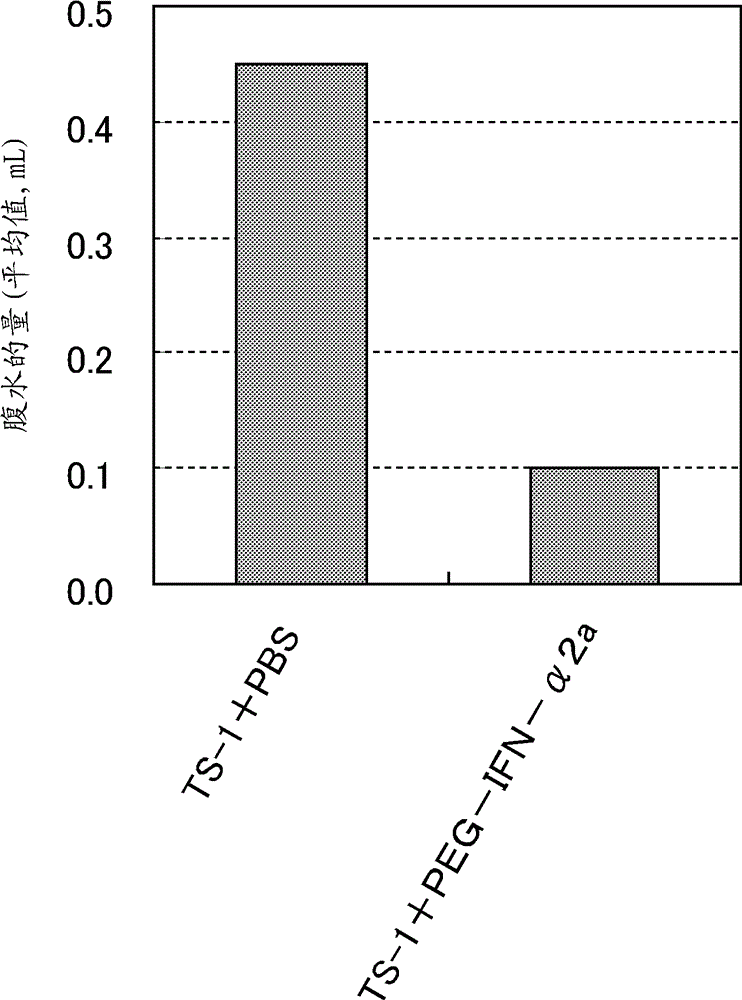
[0093] [Example 2] Effect of PEG-IFN-β on inhibiting accumulation of ascites by topical application
[0094] A mouse model of gastric cancer peritoneal metastasis was generated, and the effect of PEG-IFN-β on inhibiting ascites accumulation by topical administration was evaluated according to the method of Nakanishi et al. (Cancer Science, 2003, Vol. 94, pp. 112-118).
[0095] At 37°C and 5% CO 2 Cultured in DMEM medium containing 10% fetal calf serum and maintained in a culture box, prepared by introducing pEGFP-C1 plasmid (Clontech) into GCIY human gastric cancer cells (No. RCB0555) obtained from Cell Bank, RIKEN BioResource Center GCIY-EGFP cells. GCIY-EGFP cells were harvested with trypsin / EDTA and washed with Hank's balanced salt solution (HBSS) to prepare GCIY-EGFP cell suspension. GCIY-EGFP cell suspension (2.5 x 10 6cells / mouse) into male KSN nude mice (obtained from Shizuoka Laboratory Animal Center), and the next day according to Ohashi et al. (International Journ...
Embodiment 3
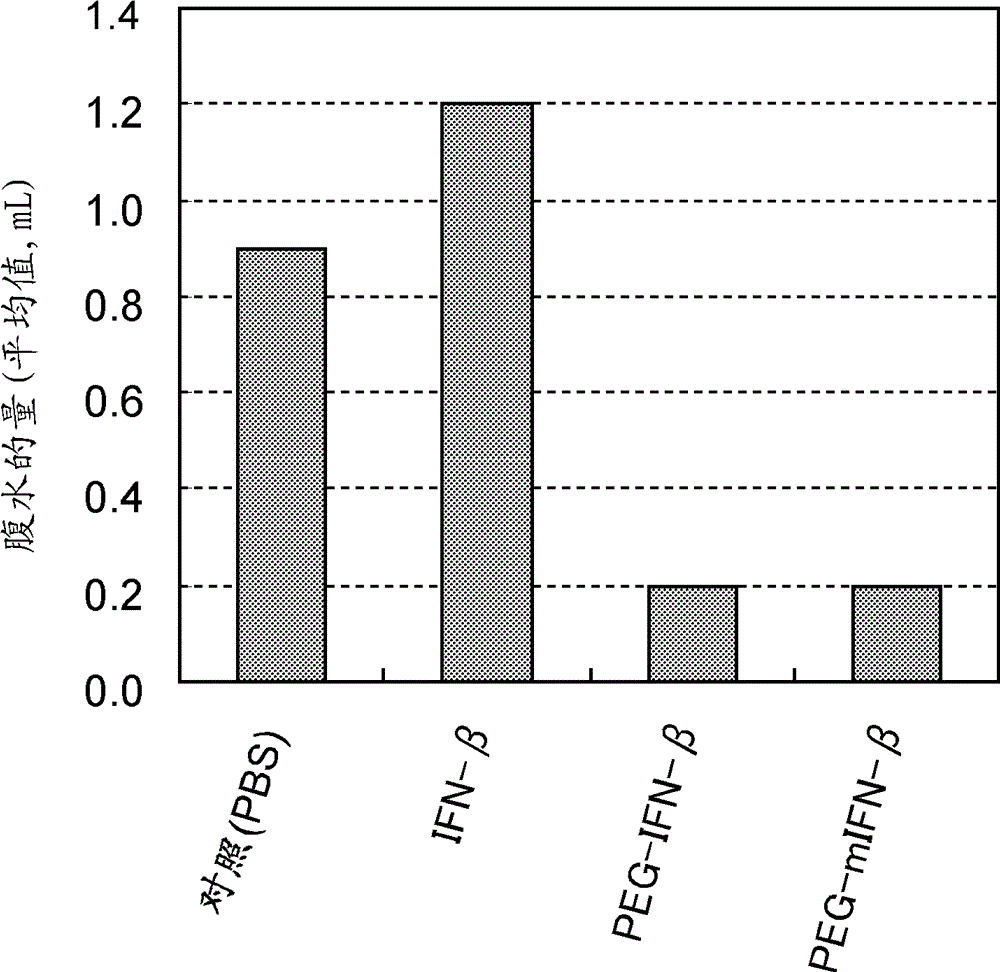
[0100] [Example 3] Effect of systemic administration of PEG-IFN-β on inhibiting accumulation of ascites
[0101] In the same manner as in Example 2, a gastric cancer peritoneal metastasis mouse model was generated by intraperitoneally transplanting human gastric cancer cells, GCIY-EGFP cells, into male KSN nude mice.
[0102] Naturally occurring human interferon-β (Feron ? ; Toray Industries, Inc.), or PEG-IFN-β or PEG-mIFN-β prepared in Example 1 were subcutaneously administered to gastric cancer peritoneal metastasis mouse model (n=6) with the amount of 5,000 U / mouse / dose dorsal region (ie by systemic administration). Administration started the day after transplantation twice a week for 4 weeks. Phosphate buffered saline (PBS) was administered to the control group in the same manner. Ascitic fluid was collected under anesthesia from the gastric cancer peritoneal metastasis mouse model 2 weeks after the last administration, and the fluid volume was measured.
[0103] The ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com