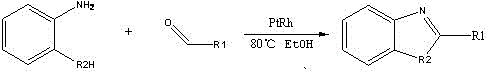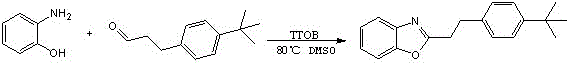Green fluorescent compound, its synthesis method and use
A green fluorescence and compound technology, applied in chemical instruments and methods, luminescent materials, organic chemistry, etc., can solve the problems of few types of yellow and green fluorescent materials, unstable chemical structure, easy crystallization or oxidation, etc., and achieve UV resistance performance. Extreme, convenient application, stable molecular structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The chemical reaction formula is as follows:
[0031]
[0032] Weigh 10mmol o-aminophenol (1.09g) and trimethylacetaldehyde (0.86g) and heat and dissolve them respectively in ethanol solution, mix them in a water bath at 60°C, add a platinum-rhodium alloy mesh as a catalyst in the solution, continue Stirring and reflux reaction for about 10 hours, a tan solid precipitate was obtained, and the solid was washed by suction filtration with ethanol several times to obtain a relatively pure product C 11 h 13 ON.
[0033] After testing, the substance has an emission wavelength of 530nm-545nm, is a green fluorescent material, and has high quantum efficiency.
Embodiment 2
[0035] The chemical reaction formula is as follows:
[0036]
[0037] Weigh 10mmol o-aminophenol (1.09g) and 4-tert-butylphenylpropionaldehyde trimethylacetaldehyde (1.90g) in DMSO (dimethyl sulfoxide) solution and heat to dissolve respectively, and place them in a water bath at 80°C Carry out a reflux reaction, add an appropriate amount of tetrabutyl titanate (TTOB) as a catalyst during the reaction, and continue the reaction for about 15 hours to obtain a gray solid precipitate. Similarly, the purer product C is obtained by repeated suction filtration and washing with alcohol. 19 h 21 ON.
[0038] After testing, the substance has an emission wavelength of 540nm-560nm, is a green fluorescent material, has a relatively stable structure, and has good ultraviolet resistance.
Embodiment 3
[0040] The chemical reaction formula is as follows:
[0041]
[0042] Weigh 10mmol of o-aminothiophenol (1.25g) and 2-chloro-4-iodopyridine-3-carbaldehyde (2.67g) in DMSO (dimethyl sulfoxide) solution, heat and dissolve respectively, and place in 80°C water bath The reflux reaction was carried out in the middle of the reaction. During the reaction, an appropriate amount of tetrabutyl titanate (TTOB) was added as a catalyst, and the reaction was continued for about 15 hours to obtain a yellow solid precipitate. Similarly, the purer product C was obtained by repeated suction filtration and washing with alcohol. 12 H6ON 2 ClI.
[0043] After testing, the substance has an emission wavelength of 520nm-540nm, is a yellow-green fluorescent material, has a relatively stable structure, and has good ultraviolet resistance.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com