Thiophene derivatives and their preparation and application in the preparation of medicines
A derivative and thiophene technology, applied to thiophene derivatives and their preparation and application in the preparation of medicines, can solve the problem of no literature report on the hypoglycemic effect, and achieve rich traditional Chinese medicine raw materials, reduce side effects, and large clinical applications. effect of value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
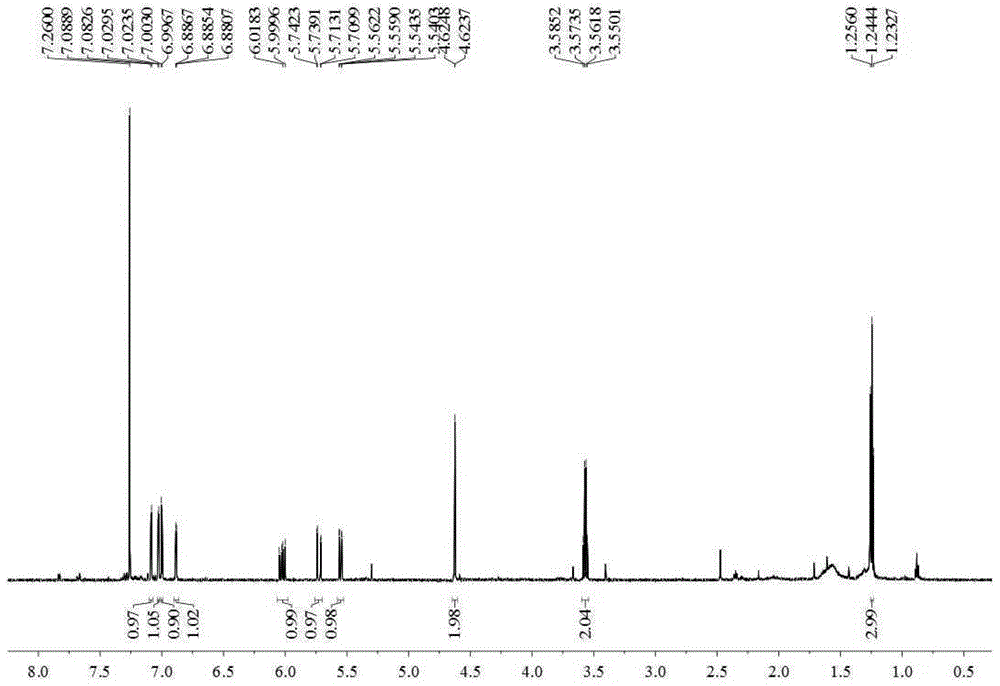
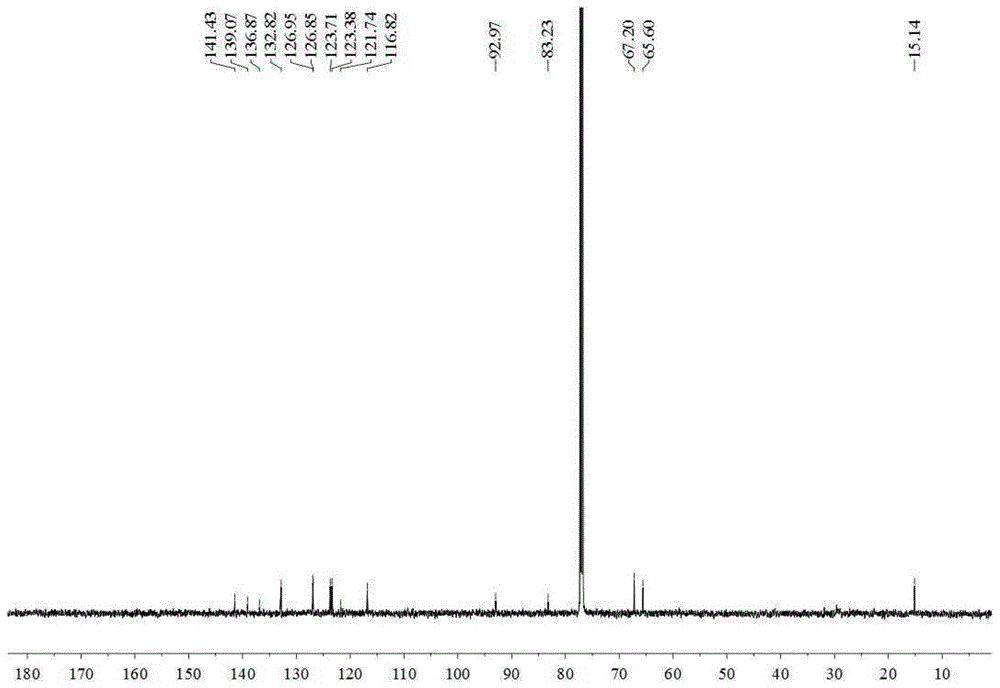
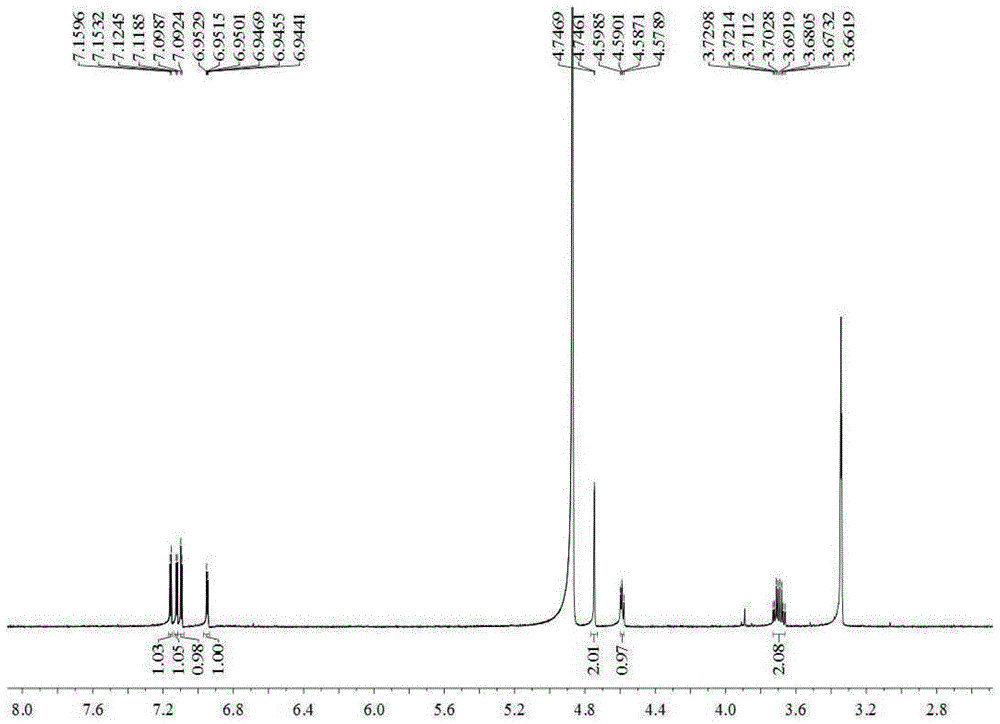
[0032] Embodiment 1 prepares thiophene derivatives
[0033] 15Kg of dried whole Eclipta chinensis, crushed, heated and refluxed with 150L of 80% ethanol for 3 times, 1 hour each time, combined the 3 extracts, and filtered to obtain the total filtrate. Take 200ml of the filtrate and concentrate under reduced pressure at 60°C to evaporate the solvent to obtain the total ethanol extract of Eclipta chinensis. The rest of the filtrate was concentrated under reduced pressure at 60°C until there was no alcohol smell, and suspended in 20L of water. Extract with petroleum ether (15L×3), ethyl acetate (15L×3), and n-butanol (15L×3) in sequence. Petroleum ether, ethyl acetate, and n-butanol layers were respectively concentrated under reduced pressure at 60°C to obtain 240 g of petroleum ether extract, 320 g of ethyl acetate extract, and 550 g of n-butanol extract.
[0034] Take 235g of petroleum ether extract, use normal phase silica gel (100-200 mesh) column chromatography, and use petr...
Embodiment 2
[0054] Example 2 In vitro hypoglycemic activity experiment
[0055] 1. Experimental drugs:
[0056] The total ethanol extract, petroleum ether fraction, ethyl acetate fraction, n-butanol fraction, and compounds 1-9 of Eclipta chinensis prepared in Example 1.
[0057] 2. Reagents and materials:
[0058] DPP-IV (dipeptidyl peptidase) was derived from the plasma of healthy volunteers; MouseTNF-α Elisa kit (BioLegend, SanDiego, USA, commercially available); DiprotinA (dipeptidin) (EnzoLifeSciences, Inc., USA, commercially available) ); other reagents (analytical grade, Sinopharm Chemical Reagent Co., Ltd., commercially available).
[0059] 3. Experimental equipment:
[0060] Automatic microplate reader (Elx800, Biotek); 96-well cell culture plate (Costar).
[0061] 4. Experimental method:
[0062] The sample to be tested was dissolved in DMSO (dimethyl sulfoxide) to obtain a stock solution of 10 mg / mL, and then tested with a concentration of 6 μg / mL. DiprotinA (distatin A) w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| control rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap