Lateral flow immunochromatographic assay product and preparation method for detecting Listeria monocytogenes
A technology of Listeria monocytogenes and immunochromatography, applied in the field of lateral flow immunochromatographic assay products and preparation for the detection of Listeria monocytogenes, can solve the problems of slow detection speed and limited on-site use, and achieve rapid detection , highly sensitive assay, strong specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
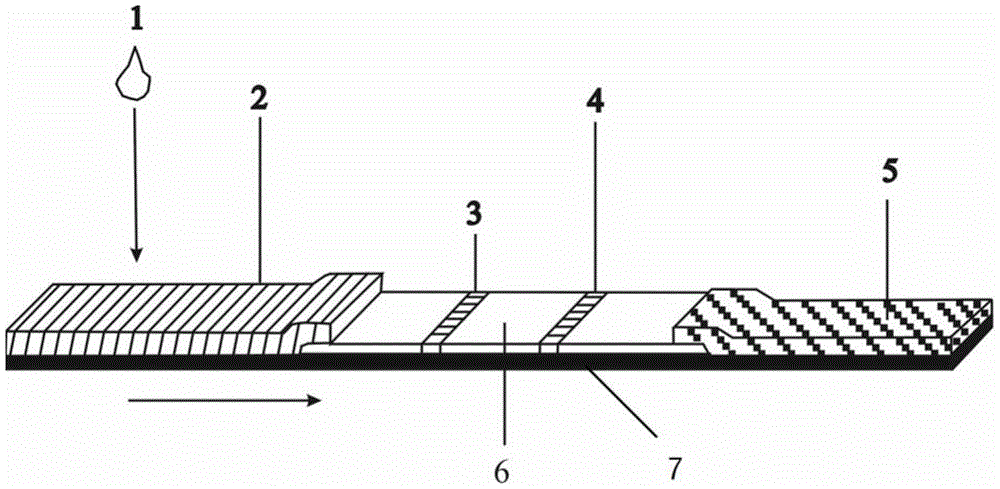
[0048] According to an embodiment of the present invention, the preparation method of the lateral flow immunochromatographic assay product comprises the following steps:
[0049] (1) Preparation of carboxyl-modified magnetic nanoparticle-labeled probes
[0050] Using suitable carboxyl-modified magnetic nanoparticles, after activating the carboxyl groups on its surface, chemical coupling is used to directionally link the Listeria monocytogenes antibody to the surface of the carboxyl-modified magnetic nanoparticles.
[0051] (2) Coating of antigen / antibody at the T line and C line in the test area
[0052] Using a film-spraying instrument, spray Listeria monocytogenes coating antibody on the T line of the test area, and spray anti-mouse IgG antibody on the C line.
[0053] (3) Coating of the labeled probe at the sample pad
[0054] Spray the anti-Listeria monocytogenes antibody labeled with magnetic microspheres (magnetic nanoparticle-labeled Listeria monocytogenes antibody) o...
Embodiment 1
[0062] Example 1, Preparation of Lateral Flow Immunochromatography Assay Paper for Detecting Listeria Monocytogenes
[0063] (1) Preparation of magnetic nanoparticles-labeled Listeria monocytogenes antibody
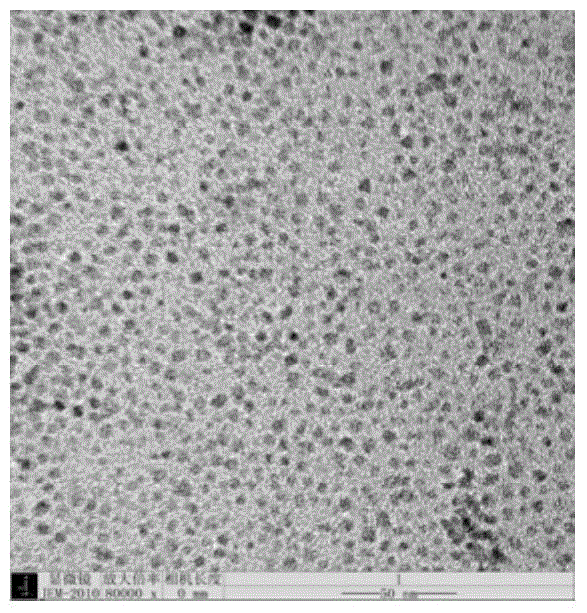
[0064] Magnetic Fe modified with polycetyl maleate (PMAH) with a saturation magnetization of 40 emu / g, an average diameter of 100 nm, and a diameter variation coefficient (CV) of 15% 3 o 4 Water-soluble nanocrystals (purchased from Shenzhen Thales Technology Co., Ltd., catalog number MP-2) are used as carboxyl-modified magnetic nanoparticles, with an affinity constant of 10 for Listeria monocytogenes. 8 m -1 The anti-Listeria monocytogenes mouse monoclonal antibody (purchased from Abnnova Company, No. MAB0532) was used as the Listeria monocytogenes antibody to be labeled, and the magnetic nanoparticle-labeled Listeria monocytogenes antibody was prepared according to the following method:
[0065] Take 2.5 mg of the above-mentioned carboxy-modified magnetic nanoparticle...
Embodiment 2
[0071] Example 2, Sensitivity detection of lateral flow immunochromatographic assay test paper for detecting Listeria monocytogenes
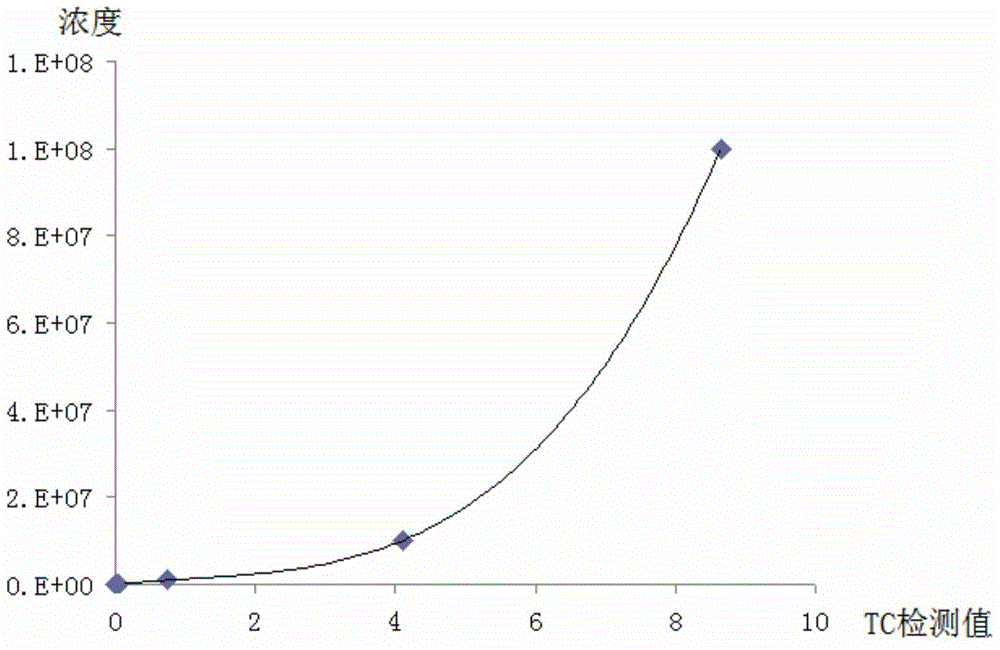
[0072] The sensitivity of the lateral flow immunochromatographic assay test paper for detecting Listeria monocytogenes in Example 1 was determined by using formaldehyde-inactivated Listeria monocytogenes as the sample to be tested.
[0073] The standard strain of Listeria monocytogenes (NICPBP54002) was taken out from the refrigerator at -20°C, activated with LEB medium, inactivated with 0.3% formaldehyde for 12 hours, and prepared into a series of concentrations (10 9 、10 8 、10 7 、10 6 、10 5 、10 4 、10 3 、10 2 , 10CFU / mL), respectively added in the lateral flow immunochromatographic assay test paper for detecting Listeria monocytogenes obtained in Example 1, and using magnetic resonance detector MAR (MagnaBioSciences, 8094-101-01&8094- 101-02) Read RMU (relative magnetic field strength). Detection steps: return the sample to be tested to...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com