Methods For Producing Mixtures Of Antibodies
A mixture, antibody technology, applied in the direction of antibodies, anti-animal/human immunoglobulins, chemical instruments and methods, etc., can solve the problems of reduced therapeutic effect and insufficient and effective treatment of monoclonal antibodies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0061] As explained above, in one aspect, the present invention relates to an in vitro method for producing a mixture comprising two or more different antibodies in a single recombinant host cell, comprising expressing in said host cell:
[0062] a) at least one nucleic acid construct encoding a common light chain, and
[0063] b) two or more nucleic acid constructs encoding heavy chains, said two or more nucleic acid constructs comprising
[0064] b1) Two or more nucleic acid constructs encoding two or more different first heavy chains, wherein the amino acid sequence of each constant region of the first heavy chains is modified such that the hinge region and the immunoglobulin Other regions of the CH region required by the protein subtype, such as the CH3 region, do not contain any disulfide bonds or covalent or stable non-covalent bonds with the same CH region in the presence of IVIG or when administered to mammals or humans. the amino acid residues of the linkage between ...
Embodiment 1
[0196] Example 1: Expression of two monovalent human antibodies with a common light chain in a single cell
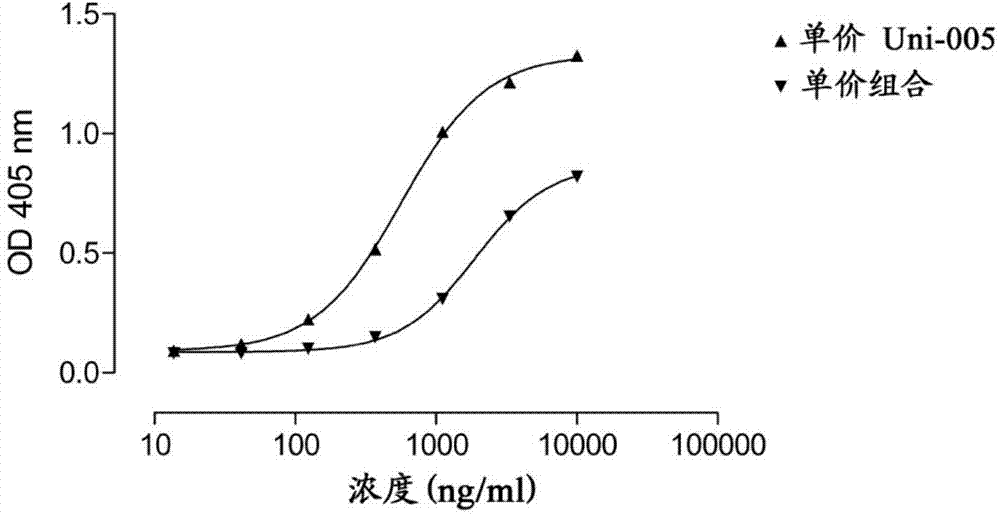
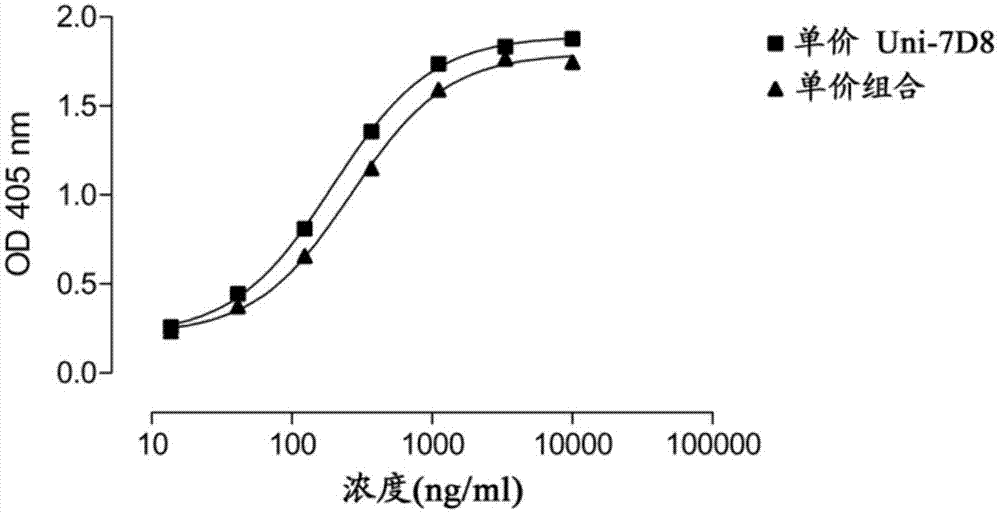
[0197] The expression vectors for the heavy chain (HC) of two antibodies, anti-CD20 antibody 7D8 (WO2004035607) and anti-CD38 antibody 005 (WO2006099875) were modified by changing the isotype to IgG4 and deleting the sequence encoding the hinge region (deleting the sequence encoding ESKYGPPCPSCP sequence) (see also WO2007 / 059782). The resulting construct was co-expressed with the light chain (LC) of 005 in HEK-293F cells (Invitrogen, as recommended by the manufacturer) by transient overexpression. Expression levels were measured by nephelometry and were within the normal expression range for this system. The potential combination of two different monovalent antibodies in the supernatant was tested by ELISA, the binding on soluble CD38 and the binding to the anti-idiotypic antibody against 7D8 was detected by ELISA as described in WO2006099875 (described in WO2004035607...
Embodiment 2
[0198] Example 2: Generation and evaluation of multiple monovalent antibodies with a common light chain in a single cell line
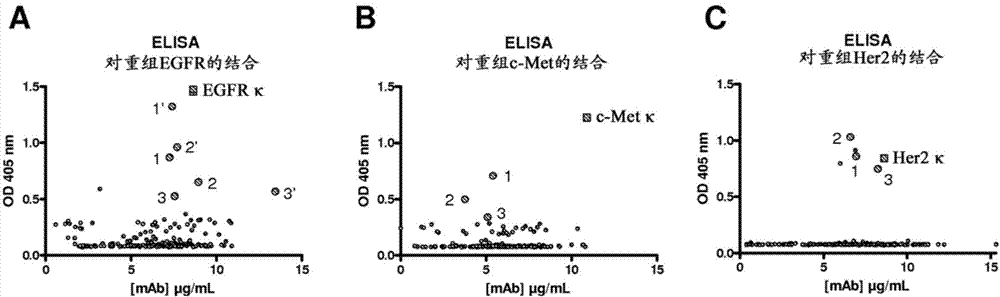
[0199] First, expression in mammals containing the constant region of a hinge-modified monovalent (hinge region E99-P110 deleted and containing substitutions F273T and Y275E in the CH3 region (SEQ ID NO: 2), as described in WO2008145140) human IgG4 antibody The sequences encoding the VH regions of a group of antibodies (specific for EGFr (clone LC1006-018, described in WO2009030239), c-Met and Her2, respectively) were cloned in a vector (pcDNA3.3, Invitrogen). To identify common light chains, each HC vector was transiently co-transfected in HEK-293F cells together with a library of expression vectors encoding a single human LCκ germline sequence. The library was included from the publicly available database VBASE (Tomlinson, I.M., Williams, S.C., Corbett, S.J., Cox, J.B.L., Winter, G., 1996. VBASE Sequence Directory. MRC Center for Protein Engineering...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com