Rivaroxaban-containing pharmaceutical preparation
A technology of rivaroxaban and pharmaceutical preparations, which is applied in the field of pharmaceutical preparations containing rivaroxaban and its preparation, achieving the effect of good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
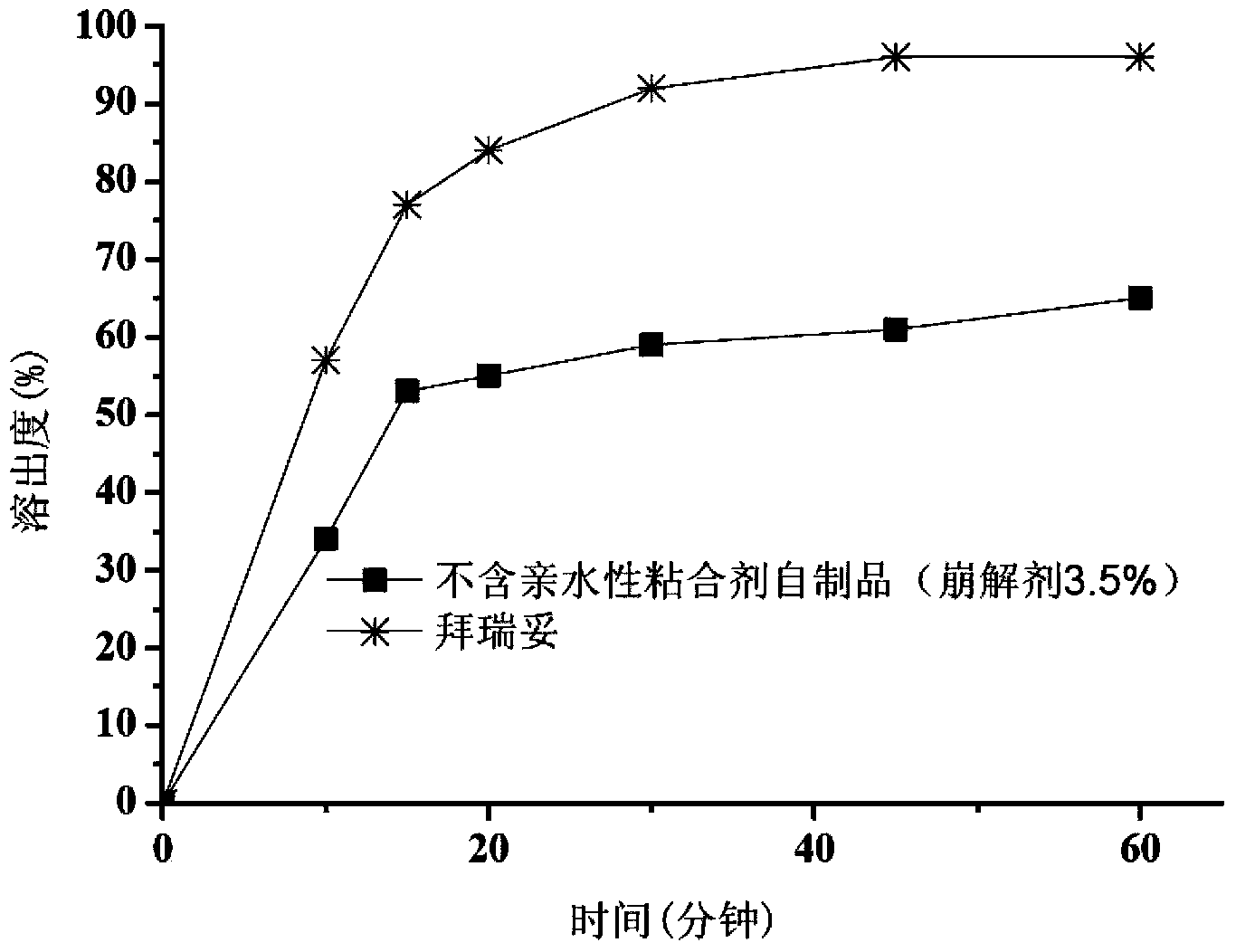
[0037] Example 1. Preparation of Tablets Without Hydrophilic Binder (3.5% Disintegrant)
[0038] 1.1 Composition of Tablets (mg / tablet)
[0039]
[0040]
[0041] 1.2 Preparation
[0042] Dissolve sodium lauryl sulfate in water. The micronized rivaroxaban was added to the solution and stirred to make it dispersed uniformly to obtain a rivaroxaban suspension. The prepared suspension was added to an excipient consisting of microcrystalline cellulose, lactose and croscarmellose sodium for wet granulation. After drying, granulate with a 24-mesh sieve and then add magnesium stearate to mix. Then, the obtained granules are compressed into tablets with a hardness of 5-9 kg by using a punching die with a diameter of 6 mm, and coated, and the weight gain of the coating is controlled to be 3%.
[0043] 1.3 Evaluation
[0044] Method: Chinese Pharmacopoeia 2010 edition two X C second method paddle method
[0045] Medium: 900ml pH4.5 acetate buffer (containing 0.2% sodium dode...
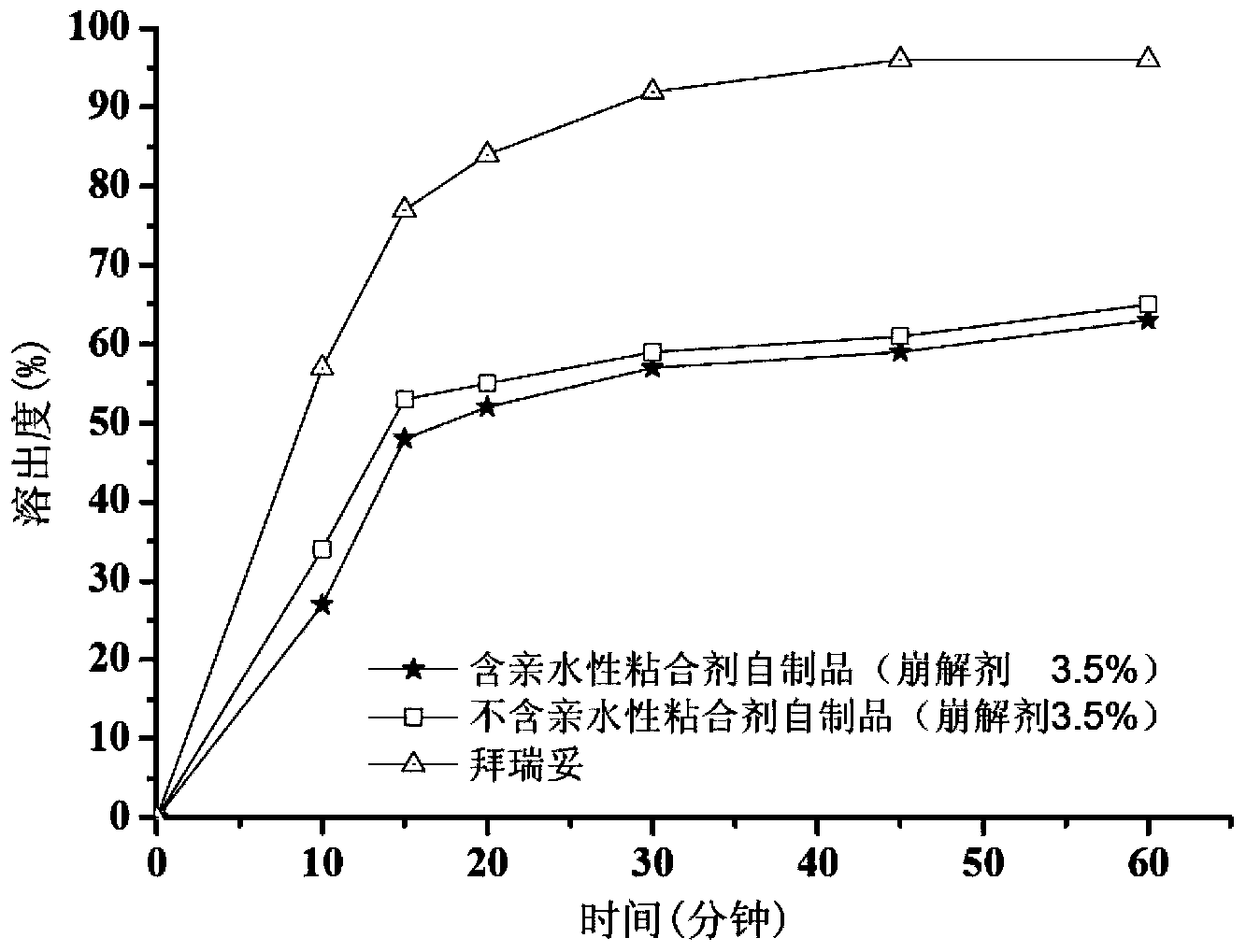
Embodiment 2
[0056] Example 2. Preparation of Tablets Without Hydrophilic Binder (7% Disintegrant)
[0057] 1.1 Composition of Tablets (mg / tablet)
[0058]
[0059] 1.2 Preparation
[0060] Dissolve sodium lauryl sulfate in water. The micronized rivaroxaban was added to the solution and stirred to make it dispersed uniformly to obtain a rivaroxaban suspension. The prepared suspension was added to an excipient consisting of microcrystalline cellulose, lactose and croscarmellose sodium for wet granulation. After drying, granulate with a 24-mesh sieve and then add magnesium stearate to mix. Then, the obtained granules are compressed into tablets having a hardness of 5-9 kg by using a punching die with a diameter of 6 mm, and are coated with a coating weight gain of 3%.
[0061] 1.3 Evaluation
[0062] The dissolution measurement method is the same as that in Example 1. The result is as image 3 As shown, although the disintegration time of this product is longer than that of the tab...
Embodiment 3
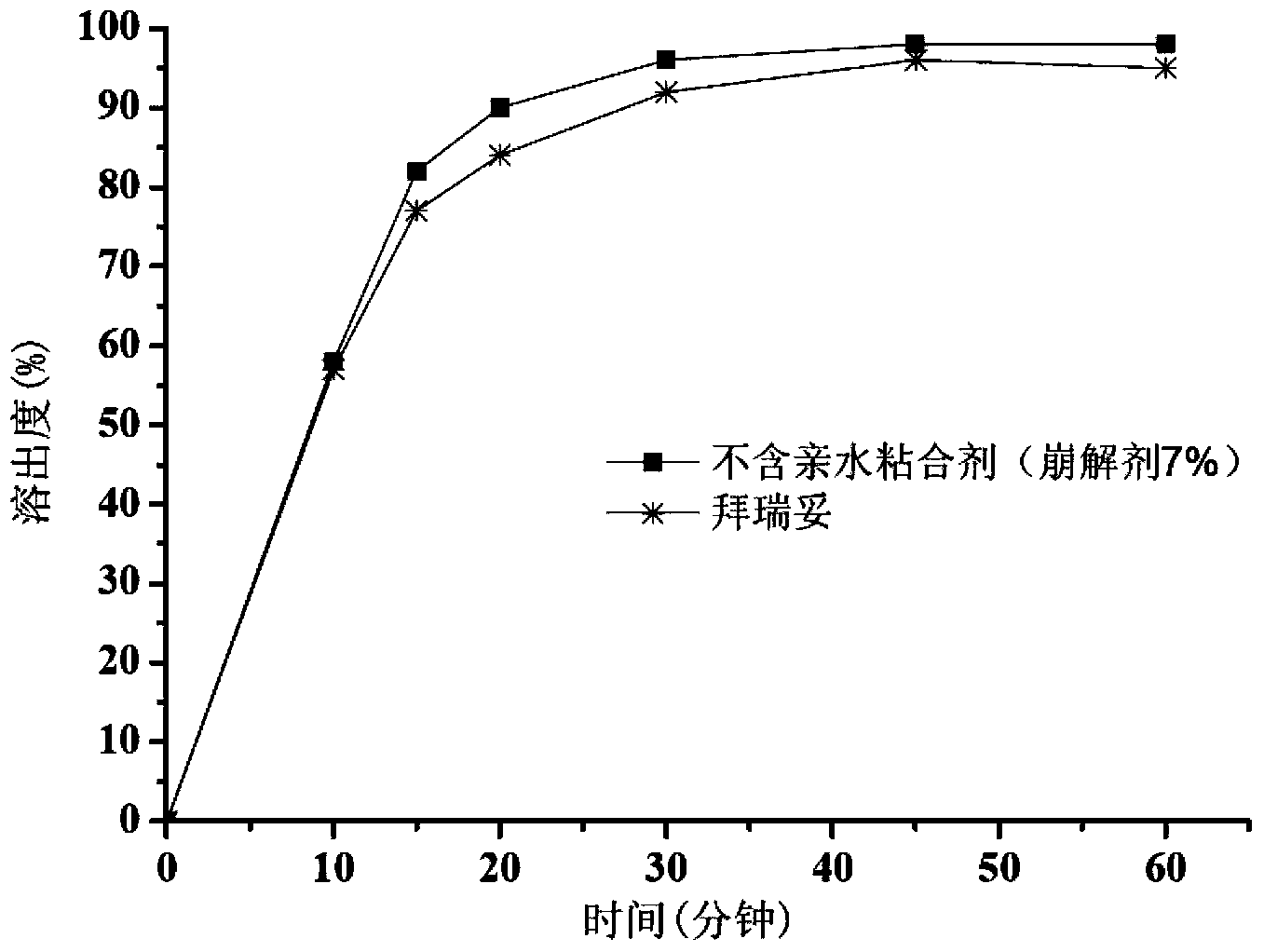
[0070] Example 3. Preparation of Tablets Without Hydrophilic Binder (15% Disintegrant)
[0071] 1.1 Composition of Tablets (mg / tablet)
[0072]
[0073] 1.2 Preparation
[0074] Dissolve sodium lauryl sulfate in water. The micronized rivaroxaban was added to the solution and stirred to make it dispersed uniformly to obtain a rivaroxaban suspension. The prepared suspension was added to an excipient consisting of microcrystalline cellulose, lactose and croscarmellose sodium for wet granulation. After drying, granulate with a 24-mesh sieve and then add magnesium stearate to mix. Then, the obtained granules are compressed into tablets having a hardness of 5-9 kg by using a punching die with a diameter of 6 mm, and are coated with a coating weight gain of 3%.
[0075] 1.3 Evaluation
[0076] The dissolution measurement method is the same as that in Example 1. The result is as Figure 5 As shown, the disintegration time of this product is 5-6 minutes, and the dissolution t...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap