Application of chiral nano-selenium materials loaded with siRNA in the preparation of antitumor drugs
An anti-tumor drug, nano-selenium technology, applied in the direction of anti-tumor drugs, drug combinations, pharmaceutical formulas, etc., can solve undiscovered problems, achieve the effects of reducing efflux, convenient and quick storage and use, and increasing drug intake
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
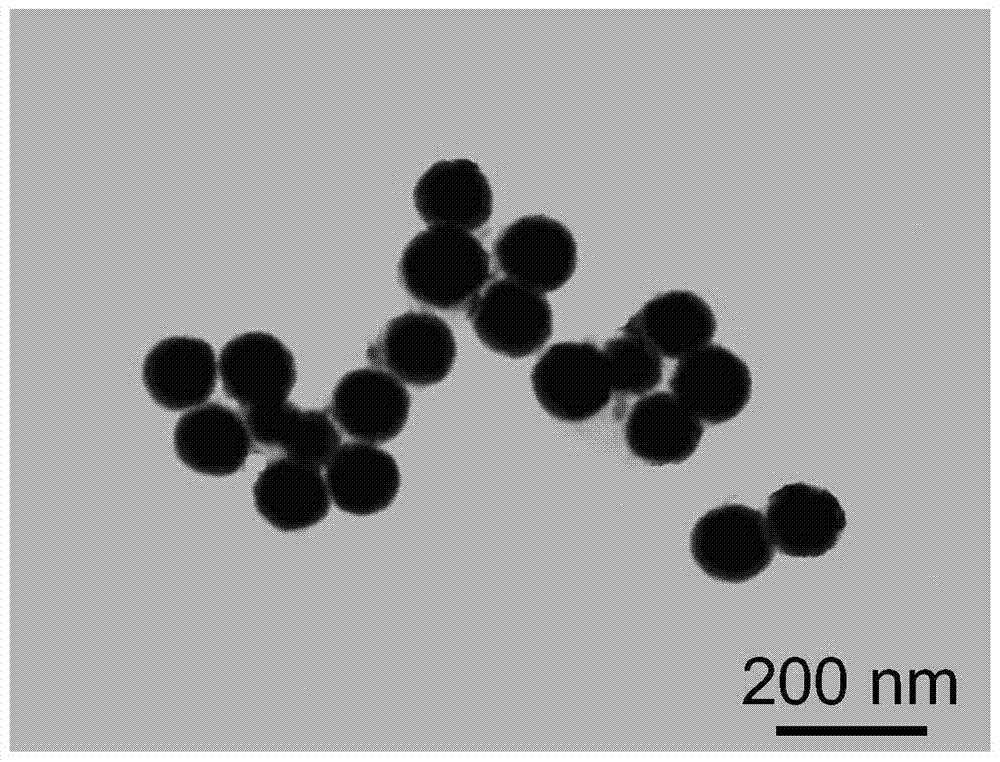
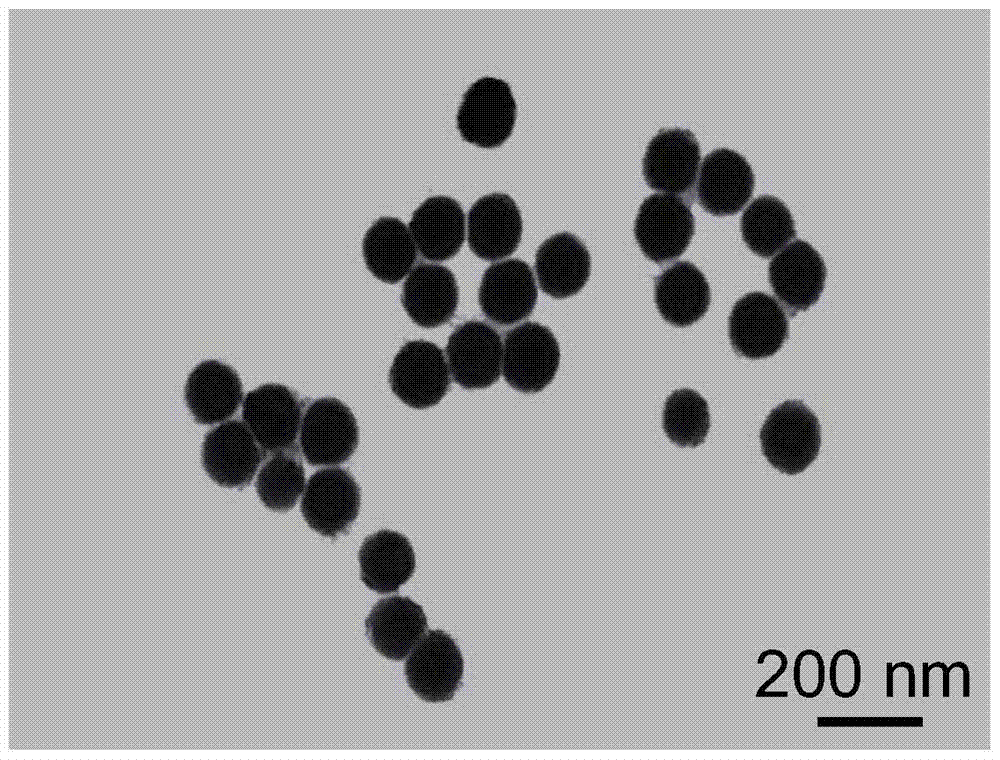
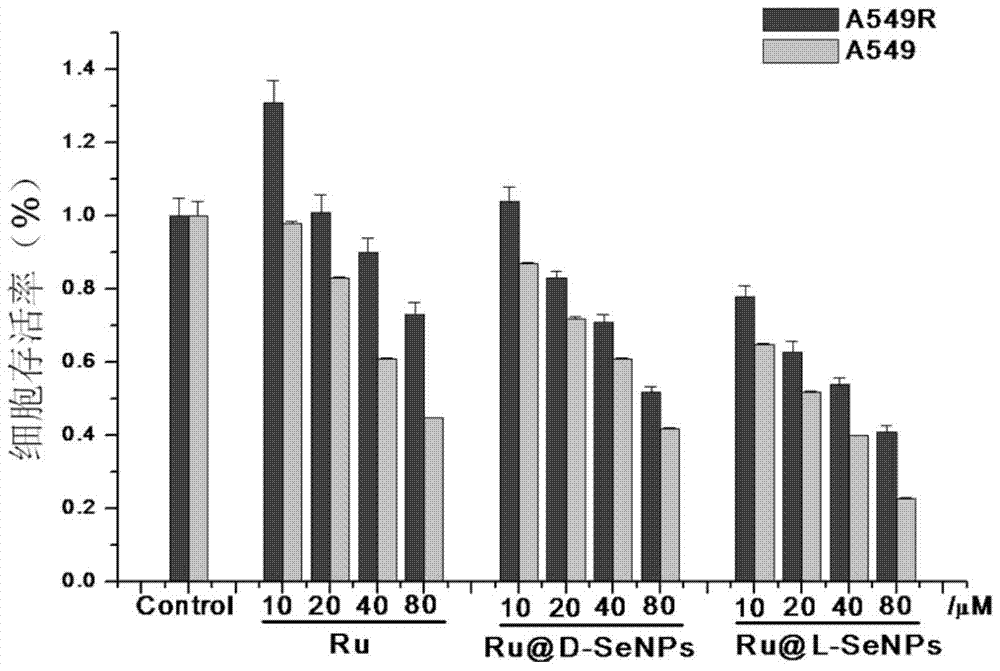
[0053] Example 1: [(phen) 2 Ru(bpibp)Ru(phen) 2 ] 4+ -L-arginine modified nano-selenium (Ru@L-SeNPs) and ((phen) 2 Ru(bpibp)Ru(phen) 2 ] 4+ -D-arginine modified nano-selenium (Ru@D-SeNPs) preparation
[0054] (1)((phen) 2 Ru(bpibp)Ru(phen) 2 ](ClO 4 ) 4 And [(bpy) 2 Ru(bpibp)Ru(bpy) 2 ](ClO 4 ) 4 Preparation method
[0055] ①cis-[Ru(phen) 2 Cl 2 ]·2H 2 Synthesis of O
[0056] Weigh RuCl 3 ·NH 2 O (0.78g, 3mmol), o-phenanthroline (1.2g, 6mmol) and lithium chloride (0.84g, 14mmol) were put in a three-necked flask, 5mL DMSO was added, and heated to reflux at 140°C under argon protection for 8 hours. After cooling to room temperature, add 25 mL of acetone, shake well and freeze overnight. Suction filtration, washing with ice water and cold acetone for 3 times, and drying in a vacuum dryer to obtain purple-black microcrystals. The crude product was recrystallized with ethanol and water (V / V=1:1), and the yield was 0.96 g (67%, calculated based on o-phenanthroline).
[0057] cis-[Ru(bpy) ...
Embodiment 2
[0068] Example 2: [(phen) 2 Ru(bpibp)Ru(phen) 2 ] 4+ -L-cysteine modified nano-selenium and [(phen) 2 Ru(bpibp)Ru(phen) 2 ] 4+ -D-cysteine modified nano-selenium preparation
[0069] (1) Ruthenium complex stock solution, Na 2 SeO 3 The preparation and concentration of the solution are the same as in Example 1;
[0070] (2) Dissolve L-cysteine and D-cysteine in purified water to prepare a 1 mol / L solution, and store it at 4°C for later use;
[0071] (3) Take 1mL Na respectively 2 SeO 3 The solution was mixed with 8mL ultrapure water to obtain solution A; 1mL of L-cysteine solution was added dropwise to solution A under constant stirring, and the pH value was maintained at 9-11 during the process; the stirring was continued for 10-30 minutes to obtain wine red Nanosol; centrifuge, discard the supernatant, wash, and dry to obtain red L-cysteine modified selenium nanoparticles. The obtained nano-selenium was resuspended to obtain 10 mL of 1 mM nano-selenium solution, and 2 m...
Embodiment 3
[0073] Example 3: [(bpy) 2 Ru(bpibp)Ru(bpy) 2 ] 4+ -L-Ascorbic acid modified nano-selenium and [(bpy) 2 Ru(bpibp)Ru(bpy) 2 ] 4+ -D-ascorbic acid modified nano-selenium preparation
[0074] (1) Ruthenium complex stock solution, Na 2 SeO 3 The preparation and concentration of the solution are the same as in Example 1;
[0075] (2) Dissolve L-ascorbic acid and D-ascorbic acid in purified water to prepare a 1mol / L solution, and store it at 4°C for later use;
[0076] (3) Take 1mL Na respectively 2 SeO 3 Mix the solution with 8 mL ultrapure water to obtain solution A; add 1 mL of L-ascorbic acid solution dropwise to solution A under constant stirring, maintaining the pH value of 3 to 5 during the process; continue stirring for 10 to 30 minutes to obtain wine red nano sol; Centrifuge, discard the supernatant, wash, and dry to obtain red nano-selenium particles. Resuspend the obtained nano-selenium to obtain 10 mL of 1 mM nano-selenium solution, and add 2 mL of [(bpy) under constant stirri...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com