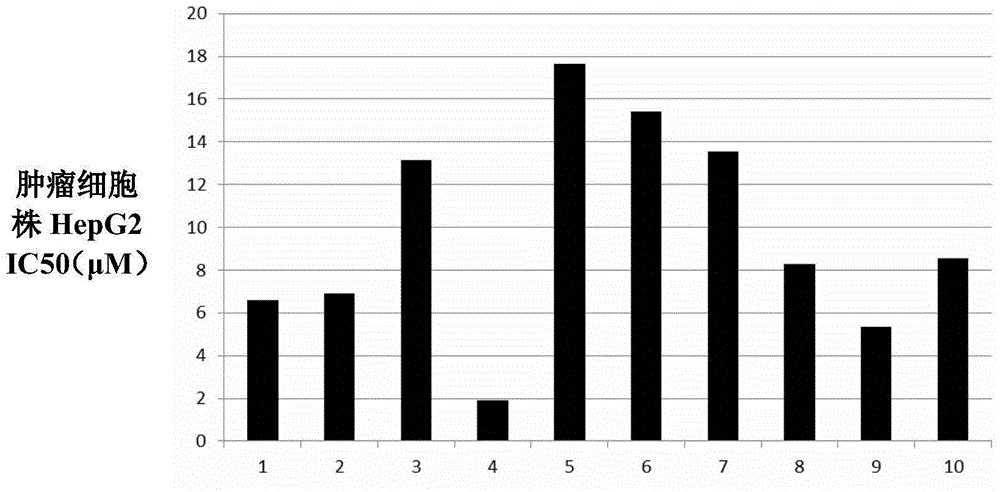
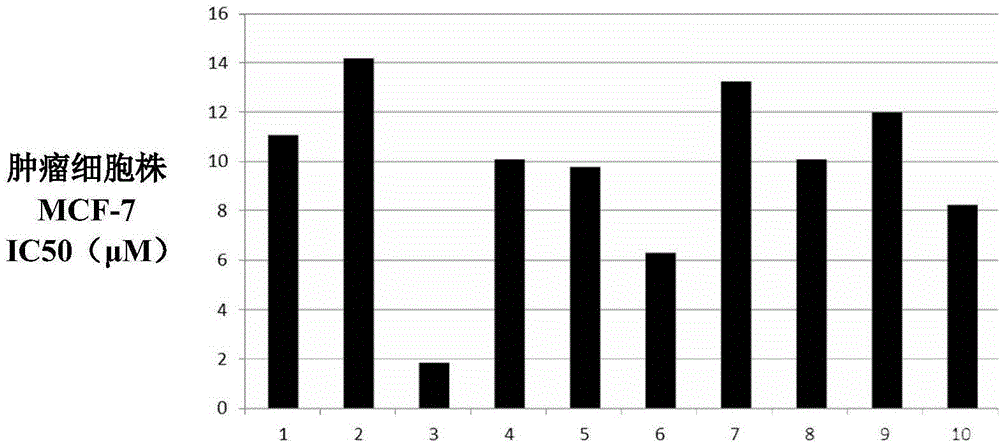
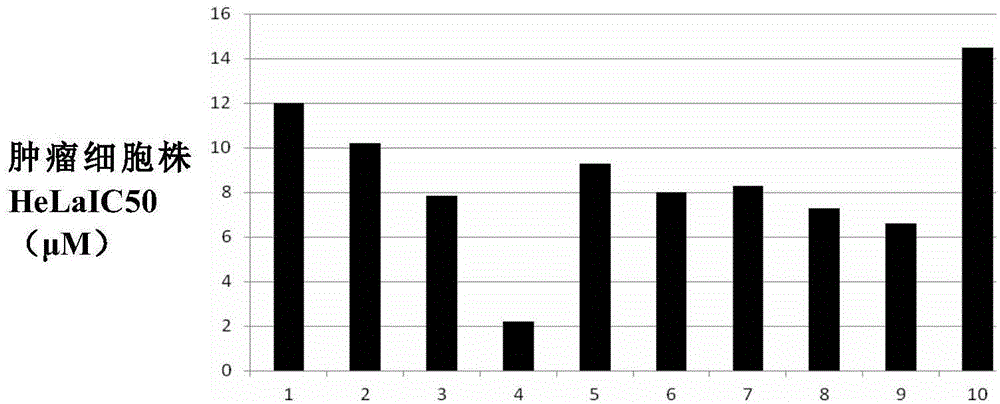
Podophyllotoxin heterocyclic lipid derivatives, and synthetic method and application thereof
A technology of podophyllotoxin and derivatives, applied in drug combination, organic chemistry, antineoplastic drugs, etc., can solve the problems of low water solubility, toxic and side effects, etc., and achieve the effect of low toxic and side effects and obvious tumor cell inhibitory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Example 1: Preparation of Podophyllotoxin Heterocyclic Lipid Derivatives
[0019] At room temperature, add podophyllotoxin, corresponding heterocyclic acid, refined dichloromethane and catalyst to a 50mL Erlenmeyer flask in sequence, the reaction is complete as detected by TLC, and the corresponding podophyllotoxin heterocyclic lipid derivatives are obtained by column chromatography. Dissolve 50mmol of podophyllotoxin and 50mmol of 2-indolecarboxylic acid in 20mL of dichloromethane, and continue to add 5%-10% of the reaction system weight N,N-dicyclohexylcarbodiimide and 4-diaminopyridine, TLC follow-up detection generates corresponding ester derivatives, add 1-1.5 times of the product weight of gel to concentrate the solvent under reduced pressure, and use dichloromethane: acetone volume ratio=100: 1 column chromatography to obtain the corresponding of podophyllotoxin ester derivatives.
[0020] Compound 1: 1 H NMR (300MHz, CDCl 3 )δ: 9.03(s, 1H), 7.69(d, J=8.1Hz, 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com