Nanotherapeutics for drug targeting
A nanoparticle, prodrug technology, applied in the field of nanotherapeutics for drug targeting, that can solve problems that have not been proposed or developed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
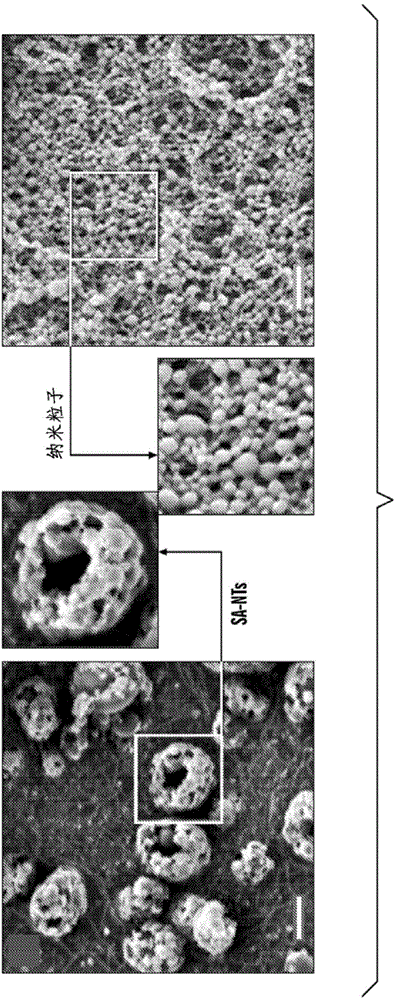
[0428] Nanoparticle Preparation: Nanoparticles (NP) were prepared from PLGA (50:50, 17 kDa, acid-terminated; Lakeshore Biomaterials, AL) using a simple solvent exchange method (26). The fluorescent hydrophobic dye coumarin-6 was incorporated into the NPs to enable visualization and quantification in this study. Briefly, 1 mg / ml polymer was dissolved in dimethyl sulfoxide (DMSO, Sigma, MO) containing 0.1 wt% coumarin, dialyzed against water at room temperature, and passed through solvent exchange and subsequently in an aqueous solvent self-assembly such that the nanoparticles are formed. The size distribution and morphology of the formed NPs were characterized using dynamic light scattering (DLS), scanning electron microscopy (SEM) and transmission electron microscopy (TEM).
[0429] Production of SA-NTs: PLGA NPs were centrifuged and concentrated to a 10 mg / ml suspension in water, and 1 mg / ml of L-leucine (Spectrum Chemicals & Laboratory Products, CA) was added. NP aggregate...
Embodiment 2
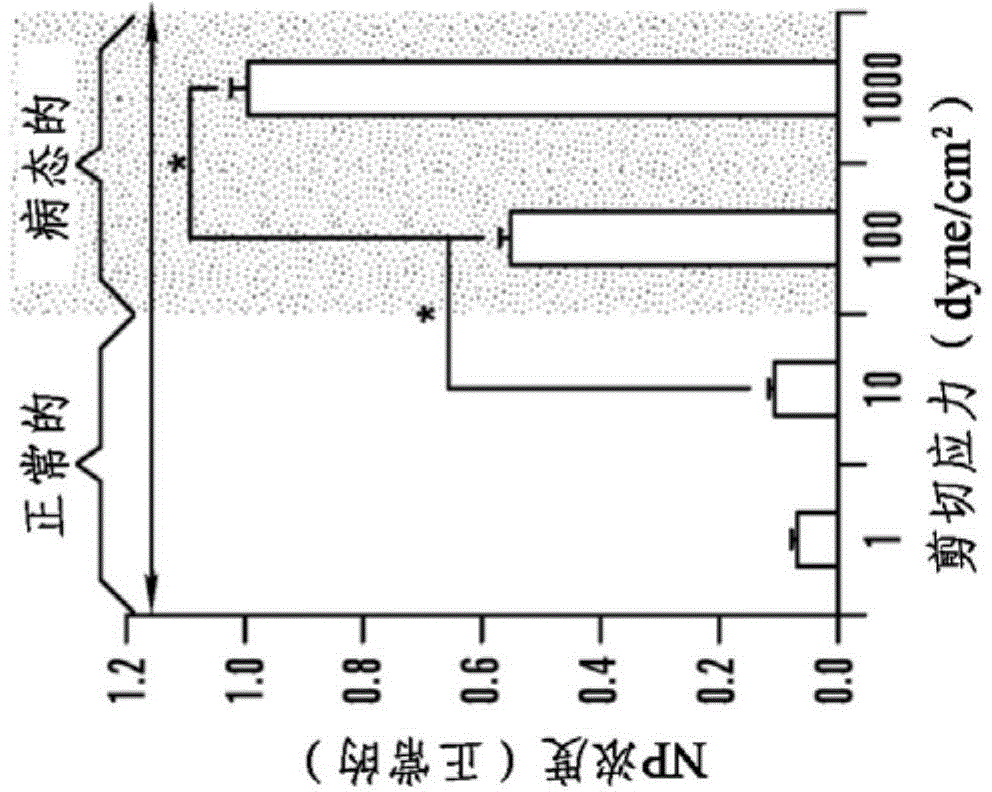
[0487] Example 2: Shear Stress Controlled Release from RBCs
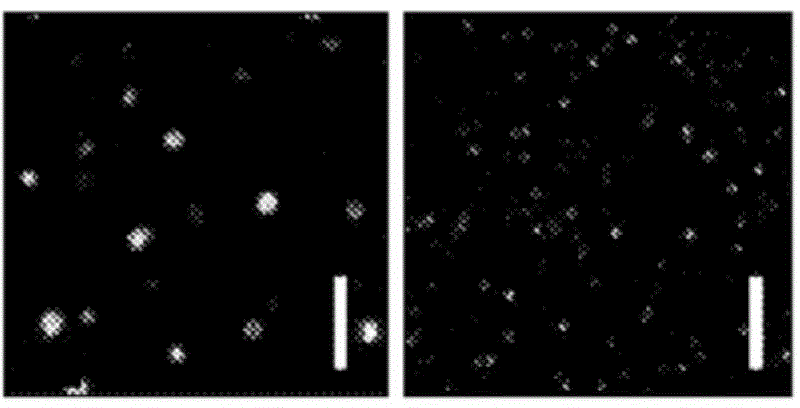
[0488] Red blood cell ghost cells were prepared using hypotonic hemolysis. Briefly, RBCs were centrifuged from blood (2000 g, 10 min) and resuspended in calcium / magnesium-free diluted PBS (1:10 volume ratio of PBS to DD water). The cells were incubated at 4°C for 15 minutes and then centrifuged (12,000 g, 10 min). This process was repeated four times. The cells were subsequently loaded with FITC-dextran by incubating the cells with 5 mg / ml of dextran in diluted PBS for 1 hour at 4°C. The cells were centrifuged, suspended in PBS buffer containing calcium / magnesium, and allowed to reseal within more than two hours in a 37°C incubator. After the reclosure procedure, the cells were washed four times in PBS to remove any residues in solution. Figure 8 Fluorescence images of FITC-dextran-loaded RBC ghost cells are shown, imaged 5 days after preparation of the FITC-dextran-loaded RBC ghost cells.
[0489] A suspensio...
Embodiment 3
[0490] Example 3: Shear Stress Controlled Release from Microcapsules
[0491] For nanocapsules, rhodamine dye-encapsulated Pluronic / polyethyleneimine (F127 / PEI) nanocapsules (S.H.Choi, S.H.Lee & T.G., Park , Temperature-sensitive pluronic / poly(ethyleneimine) nanocapsules for thermally triggered disruption of intracellular endosomal compartment, Biomacromolecules. 2006 Jun; 7(6):1864-70). Briefly, Pluronic F127 was activated with p-nitrophenyl chloroformate in toluene for 24 hours at room temperature. The product was precipitated in ether and passed through 1 H NMR characterization. To prepare the nanocapsules, 30% of activated F127 and a small amount of hydrophobic dye (rhodamine) were dissolved in dichloromethane (1 ml), then added dropwise to 10 ml of aqueous PEI solution (7.5w / v, pH 9) . The mixture was stirred at room temperature for about 1 hour to obtain nanocapsules / microcapsules, and the entrapped dichloromethane was distilled off. The microcapsules obtained are s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com