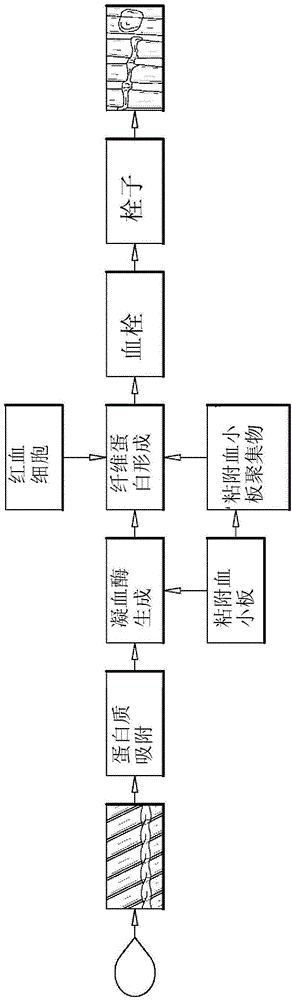
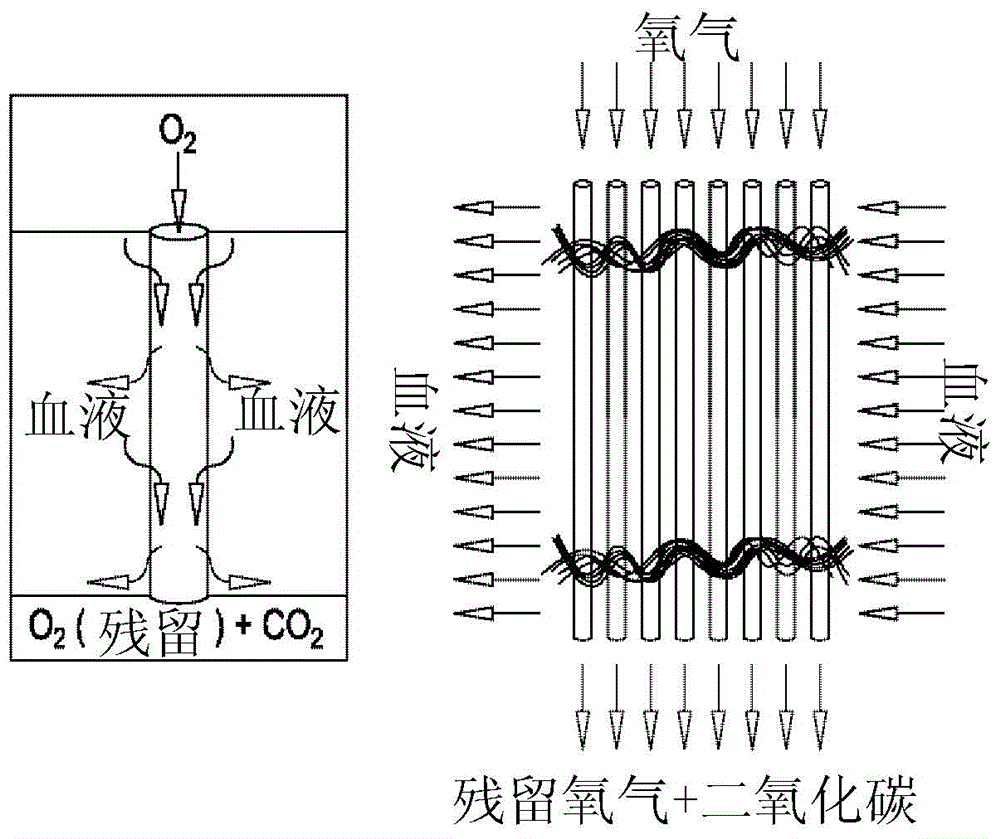
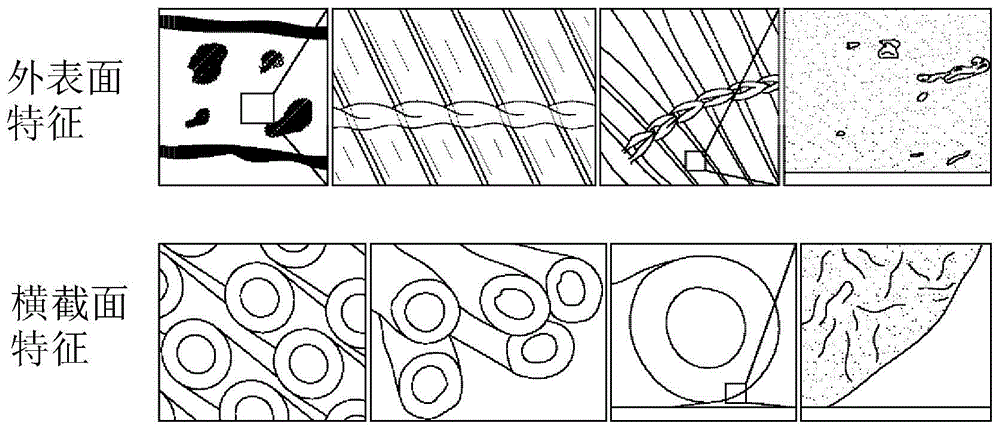
Biocompatible coating compositions
A coating composition and biocompatibility technology, applied in the field of biocompatible coating composition, can solve the problem that anticoagulant properties cannot be used for long-term support
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0053] Preparation of primer solution:
[0054] According to a non-limiting exemplary embodiment, the surface to be coated may first be primed with the PVP solution. Specifically, a solution comprising 0.5-0.9% by weight of poly-N-vinyl-2-pyrrolidone (PVP) in alcohol was prepared separately for the application of the initial surface modification and this solution was referred to as the 'primer solution' .
[0055] Method of coating a surface or device:
[0056] Test fiber samples of hollow fiber membranes can be coated directly with a coating solution of Quat-heparin complex in an alcohol-rich solvent or primed with PVPV in alcohol prior to coating surfaces or devices , followed by a second coat using the coating solution mentioned above.
[0057] Accordingly, provided herein are methods comprising coating a surface with a primer solution comprising PVP followed by coating said surface with a QUAT solution comprising a quaternary ammonium salt and heparin.
[0058] The act...
Embodiment 1
[0074] The amount of immobilized heparin on the PVP heparin (PVP-HC) coated surface was compared to the heparin coated surface (HC) and the Corline heparin surface (CHS). Mix 200 μL of AT-III and 25 μL of heparin at a concentration of 0.5-15 μg / mL and incubate at 37° C. for 2 minutes. Then add 200 μL of FX a Mix into solution and incubate at 37°C for 1 minute. Next, add 200 μL of spectrozyme FX a Mix into solution and incubate at 37°C for 5 minutes. Add 200 µL of glacial acetic acid and mix thoroughly. Heparin absorption was then measured at 405 nm, thus quantifying the activity of heparin immobilized on the fiber surface. Figure 4 is a standard straight line depicting the activity of known amounts of heparin with respect to absorbance readings determined by spectrophotometric techniques. The amount of immobilized heparin from CHS, HC and PVP-HC coated fiber samples is indicated on the standard curve. Figure 4 It was demonstrated that PVP-HC has a higher amount of immo...
Embodiment 2
[0076] HFMs each coated with different exemplary coatings of the present invention were tested to determine their biocompatibility and ability to hinder protein absorption, platelet adhesion, and thrombus deposit formation and adhesion. These were then compared to uncoated HFM bundles and other commercially available biocompatible coatings on HFM. Table 2 provides details of the different coatings.
[0077] Table 2
[0078]
[0079] Materials and methods
[0080] HFM with coating of the present invention
[0081] The efficacy of HFM with the QUAT coating of the present invention and the PVP-QUAT coating was compared to uncoated HFM and HFM coated with other commercially available coatings. QUAT and PVP-QUAT coated HFMs were prepared using the method described above. Before the PVP-QUAT and QUAT coating processes, the HFM fibers were mechanically blocked at both ends to prevent blood from entering through the lumen of the HFM.
[0082] Commercial Coated and Uncoated Hol...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap