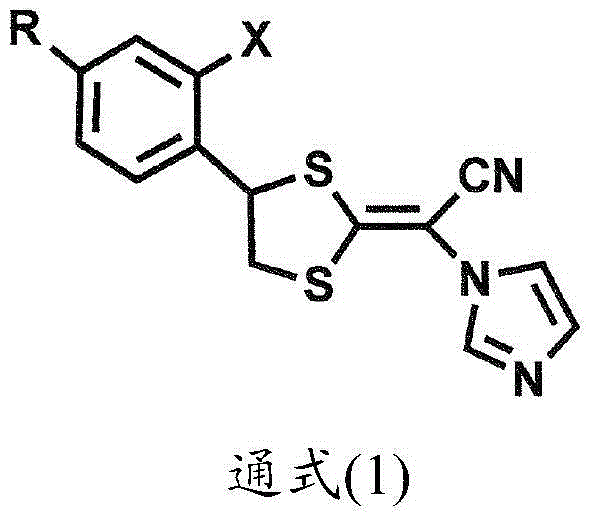
Pharmaceutical composition for diseases caused by pathogenic microorganisms such as aspergillus
A composition and protozoan technology, which can be used in drug combinations, respiratory diseases, antibacterial drugs, etc., and can solve the problems of non-protozoan effect and anti-chlamydia effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
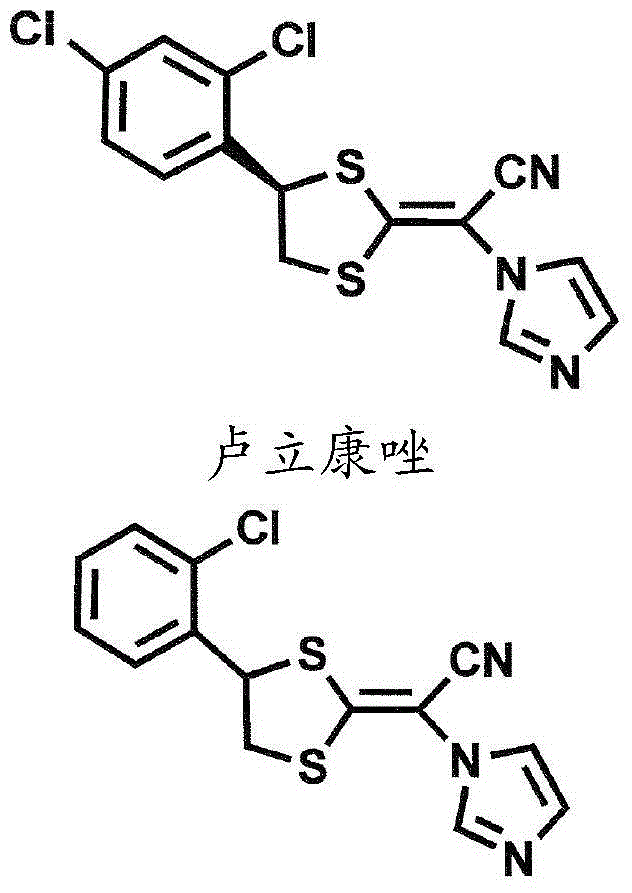
[0068] The effect of luliconazole among the compounds represented by the general formula (1) on Trichomonas vaginalis was investigated. In other words, the 5 x 10 6 Three clinically isolated Trichomonas vaginalis cells were inoculated in Trichomonas medium F (6.5 mL, contained in a tube) produced by Fujiyakuhin Co., Ltd. containing neutral red as a marker, and then Precultivation was carried out for 72 hours (preculture). Trichomonas growth was confirmed, acid was actively produced, and the neutral red turned yellow. Thereafter, each 100 µL of the preculture was added to Trichomonas medium F, to which 0.5 mL of the test solution was added for main culture. In this case, the number of protozoa in the solution of the preculture was 1.5 x 10 5 cells / mL. Three series of test solutions were prepared in which luliconazole concentrations were 200 μM (final concentration: 35.2 μM), 100 μM (final concentration: 17.6 μM) and 50 μM (final concentration: 8.8 μM) when luliconazole was ...
Embodiment 2
[0072]The same or equivalent study as in Example 1 was performed, with luliconazole replaced by lanoconazole. Therefore, it is clear that lanoconazole also inhibits the growth of Trichomonas as well as luliconazole. It has been revealed that lanoconazole is a clinically applicable substance that inhibits the growth of Trichomonas in addition to metronidazole. In addition, the minimum growth inhibitory concentration (MIC) was revealed to be around 17.6 μM.
[0073] Table 2
[0074] final concentration
Embodiment 3
[0076] In vitro antifungal activity against Candida albicans
[0077] The minimum growth inhibitory concentration (MIC) was measured by means of the broth microdilution method (two serial dilutions of the drug) based on the use of BPMI 1640 medium (pH 7.0) buffered with 0.165M morpholinopropanesulfonic acid. Dispense 100 µL of the test microorganism yeast cell / sterile saline suspension (1 to 5 x 10 3 cells / mL) and 100 μL of culture medium pre-supplemented with each compound and the culture medium without compound added as a control were distributed into each well of a flat-bottomed microculture plate. After culturing at 35°C for 48 hours, the culture turbidity of each well was measured at 630nm to determine the minimum growth inhibitory concentration (MIC 80 : μg / mL), which is the minimum concentration of compound (measured as a suspension) that exhibits 80% growth inhibition relative to the growth of microorganisms in control cultures. The results are shown in Table 3. It ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com