Aralkyl- and aryloxyalkyl-substituted epithelial sodium channel blocking compounds
A compound and alkoxy technology, applied in metabolic diseases, drug combinations, skin diseases, etc., can solve problems such as limited weight, short half-life, and weak efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
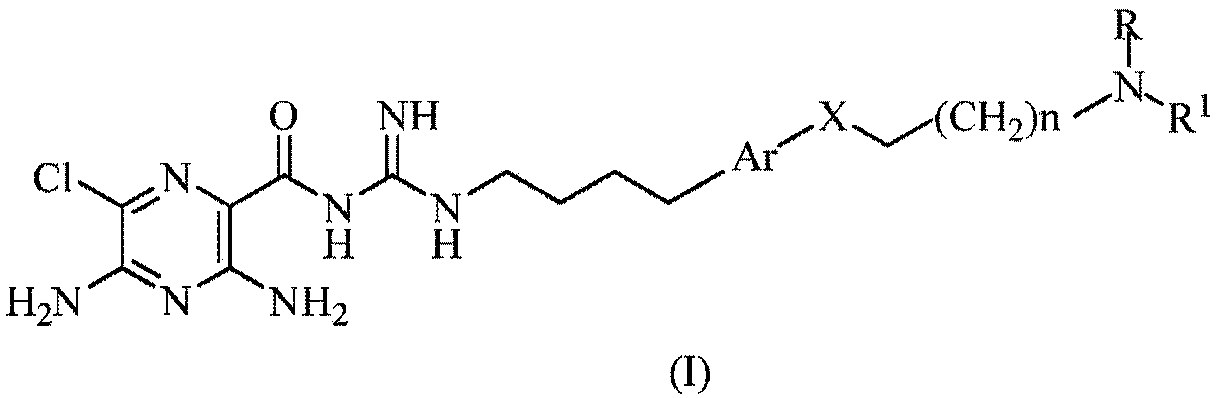
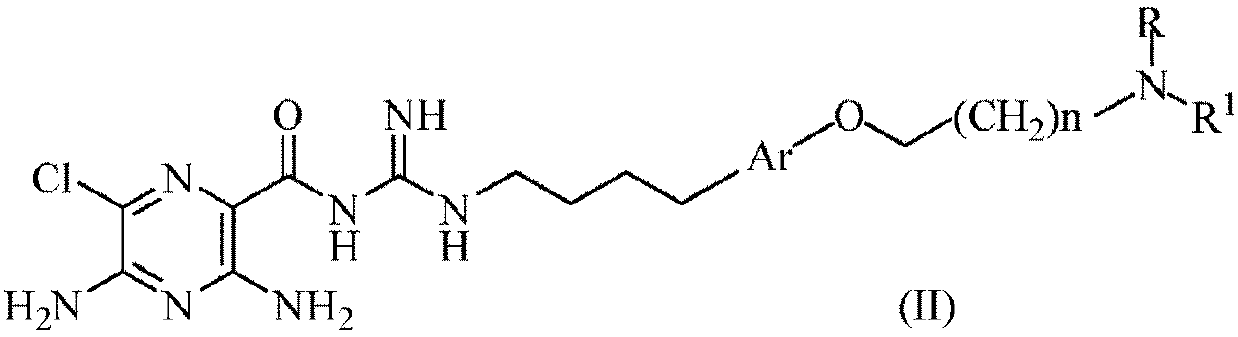
[0063]
[0064] where, in each case,
[0065] n is an integer selected from 1, 2, 3, 4, 5 and 6;
[0066] R is for -CH 2 -(CHOH) r -CH 2 OH, wherein r is an integer selected from 1, 2, 3, 4, 5 or 6; and
[0067] R 1 selected from -(CH 2 ) q -Y or -(CH 2 ) q -O-Y;
[0068] q is an integer independently selected in each instance from 1, 2, 3, 4, 5 or 6;
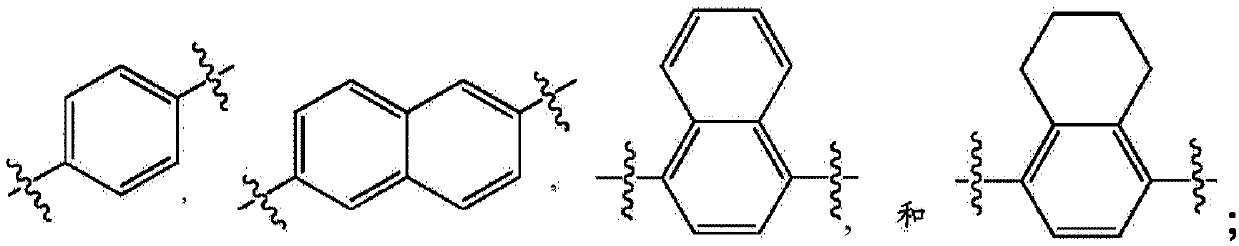
[0069] Y is a phenyl, naphthyl or pyridyl ring, wherein each phenyl, naphthyl or pyridyl ring is substituted with 0, 1, 2 or 3 substituents independently selected from: halogen, -OH, -CN , -NO 2 , -NH 2 , -NH(C 1 -C 6 Alkyl), -N(C 1 -C 6 alkyl) 2 、C 1 -C 6 Alkyl, C 1 -C 6 Alkoxy and -CF 3 .
[0070] In each of the above described embodiments for formulas (III), (IV), (V) and (VI) or pharmaceutically acceptable salts thereof, there is yet another embodiment wherein:
[0071] n is an integer selected from 2, 3 and 4;
[0072] R is CH 2 -(CHOH) r -CH 2 OH, wherein r is an integer selected from 1, 2, 3,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com