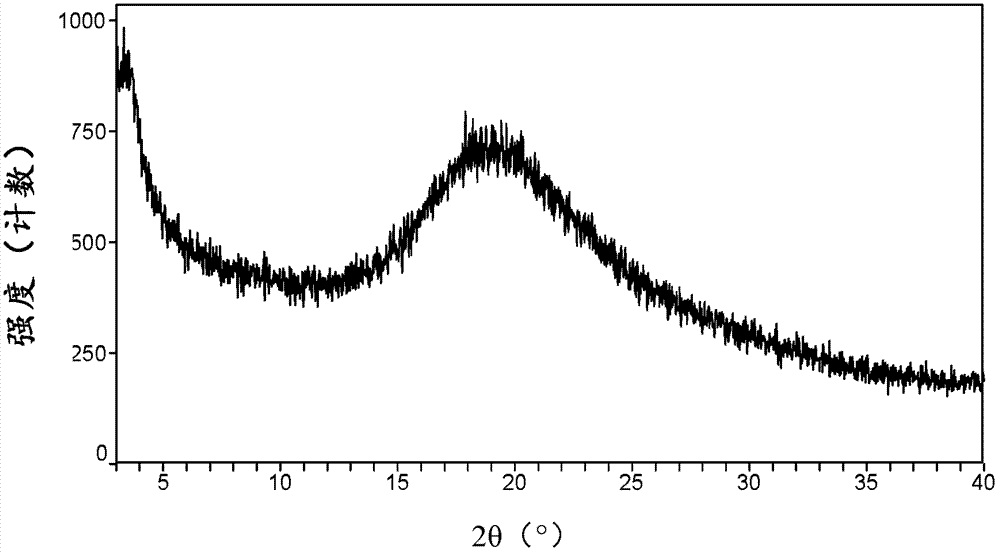
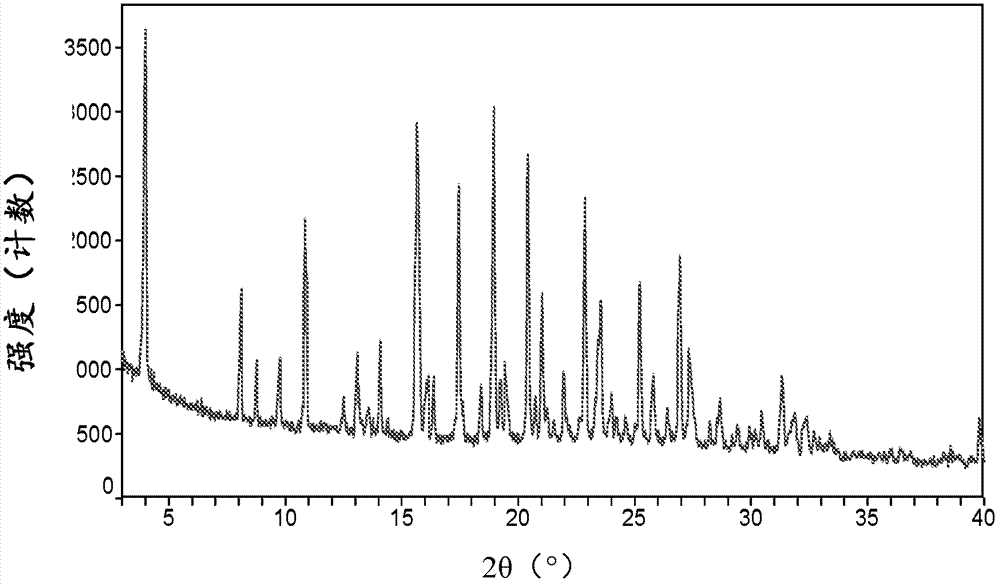
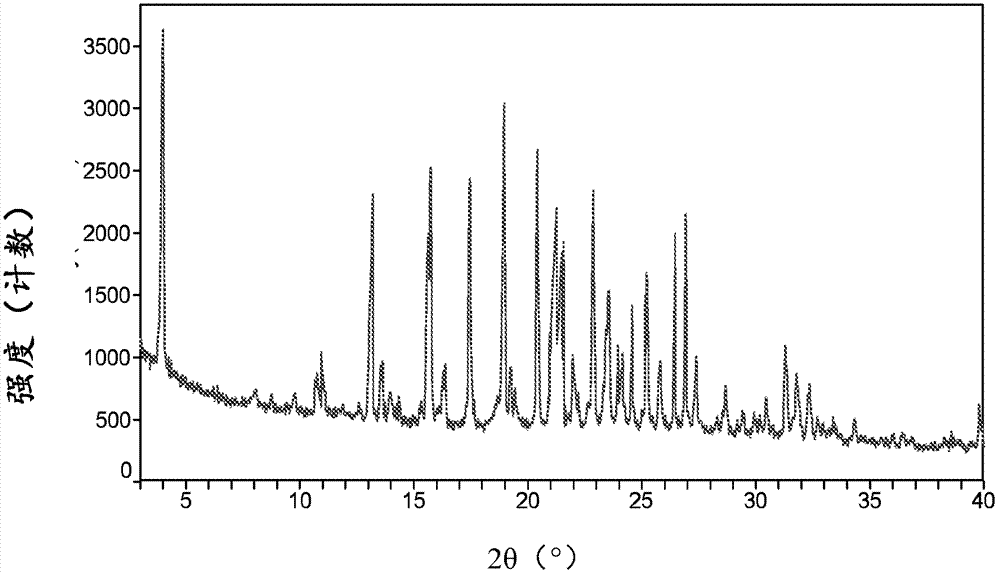
Canagliflozin monohydrate and its crystal forms, their preparation method and use
A technology of monohydrate and canagliflozin, which is applied in the field of canagliflozin monohydrate and its crystal form, can solve the problem of poor stability, poor production reproducibility and difficulty in repetition of canagliflozin hemihydrate crystal form hH1 And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0133] Preparation Example 1 (preparation of known canagliflozin)
[0134] The known canagliflozin can be prepared according to the method described in Example 1 of the patent document US7943788B2 or according to the following method.
[0135]
[0136]The specific preparation method is: 5-bromo-1-[5-(4-fluorophenyl)-2-thienylmethyl]-2-methylbenzene (2.65 grams) is dissolved in tetrahydrofuran (20 milliliters)-toluene ( 40 mL), and the mixture was cooled to -78°C under argon atmosphere. To the mixture was added n-butyllithium (2.44M hexane solution, 2.9 mL) dropwise, and the mixture was stirred at the same temperature for 30 minutes. Then, a solution of 2,3,4,6-tetra-O-trimethylsilyl-D-glucono-1,5-lactone (2.3 g) in toluene (50 ml) was added dropwise, and at the same temperature The mixture was stirred for an additional 1 hour, resulting in lactoalcoholate. Without isolation of the compound, a solution of methanesulfonic acid (1.0 ml) in methanol (50 ml) was added to th...
preparation example 2
[0139] Preparation example 2 (preparation of known canagliflozin crystal form A)
[0140] The known crystal form A of canagliflozin can be prepared according to the method described in Example 9 of patent document WO2009 / 035969A1.
[0141] 9.7 g of canagliflozin prepared in Preparation Example 1, 0.6 ml of water and 27.5 ml of ethyl acetate were added to a 100 ml three-neck round bottom flask. The resulting solution was heated to 35°C with stirring under argon. Heptane was added dropwise until the solution became cloudy for a total of 16.0 mL of heptane. After stirring at 35°C for 2 hours, add 3.0 ml of heptane, continue to stir for 30 minutes, filter under reduced pressure, wash the filter cake with 5.0 ml of 56% ethyl acetate / 44% heptane solution, and dry the filter cake at 40°C for 24 hours to obtain Canagliflozin Form A.
[0142] 1 H-NMR (CD 3 OD): 2.32(s, 3H), 3.35-3.53(m, 4H), 3.71(d, 1H, J=11.9Hz), 3.90(d, 1H, J=11.9Hz), 4.13(d, 1H, J =9.3Hz), 4.17(s, 2H), 4.9(s...
preparation example 3
[0144] Preparation example 3 (preparation of known canagliflozin hemihydrate crystal form hH1)
[0145] The known crystal form of canagliflozin hemihydrate hH1 can be prepared according to the method described in Example 1 of the patent document US7943582B2 or according to the following method.
[0146] The specific preparation method is: take 1 gram of canagliflozin prepared in Preparation Example 1, add 2.2 ml of water: acetonitrile (10:1) mixed solvent, stir at room temperature for 24 hours, filter under reduced pressure, and dry at 40°C for 24 hours to obtain canagliflozin Ggliflozin hemihydrate crystal form hH1.
[0147] 1 H-NMR (DMSO-d 6 )2.26(s, 3H), 3.13-3.28(m, 4H), 3.44(m, 1H), 3.69(m, 1H), 3.96(d, 1H, J=9.3Hz), 4.10(m, 1H), 4.15(m, 1H,) 4.43(t, 1H, J=5.8Hz), 4.72(d, 1H, J=5.6Hz), 4.92(d, 2H, J=4.8Hz), 6.80(d, 1H, J = 3.5Hz), 7.11-7.15 (m, 2H), 7.18-7.25 (m, 3H), 7.28 (d, 1H, J = 3.5Hz), 7.59 (dd, 2H, J = 8.8, 5.4Hz). Shown as canagliflozin hemihydrate.
[01...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com