Recombinant adeno-associated virus vector carrying human papillomavirus type 16 multi-point mutant e7mm antigen gene and its construction method and application
A human papilloma virus, hpv-16e7mm technology, applied in the application field of preparing anti-HPV-16 infection and related disease treatment drugs, and can solve problems such as safety risks
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
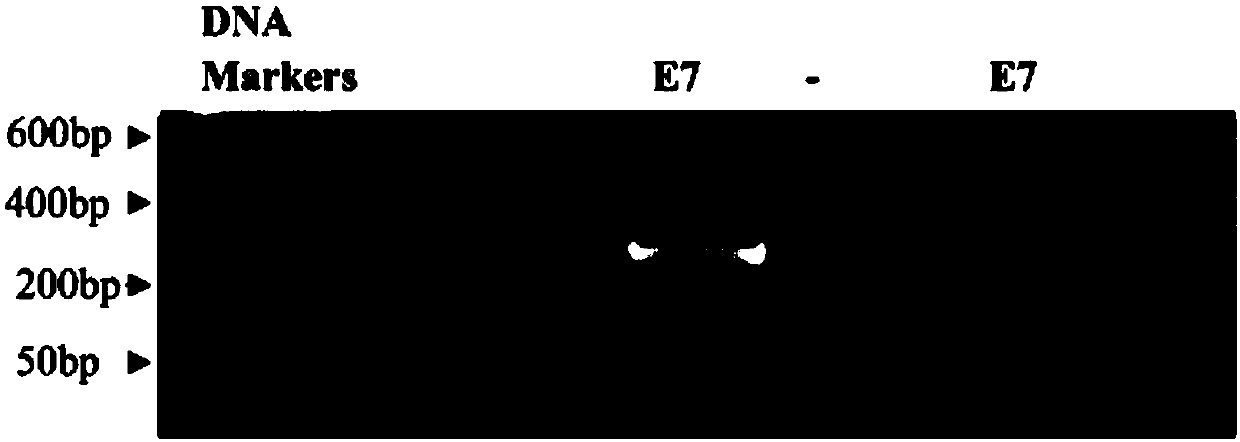
[0072] Example 1, Construction of recombinant adeno-associated virus vectors AAV / HPV-16 E7 and AAV / HPV-16 E7 mm
[0073] 1. Materials and their sources:
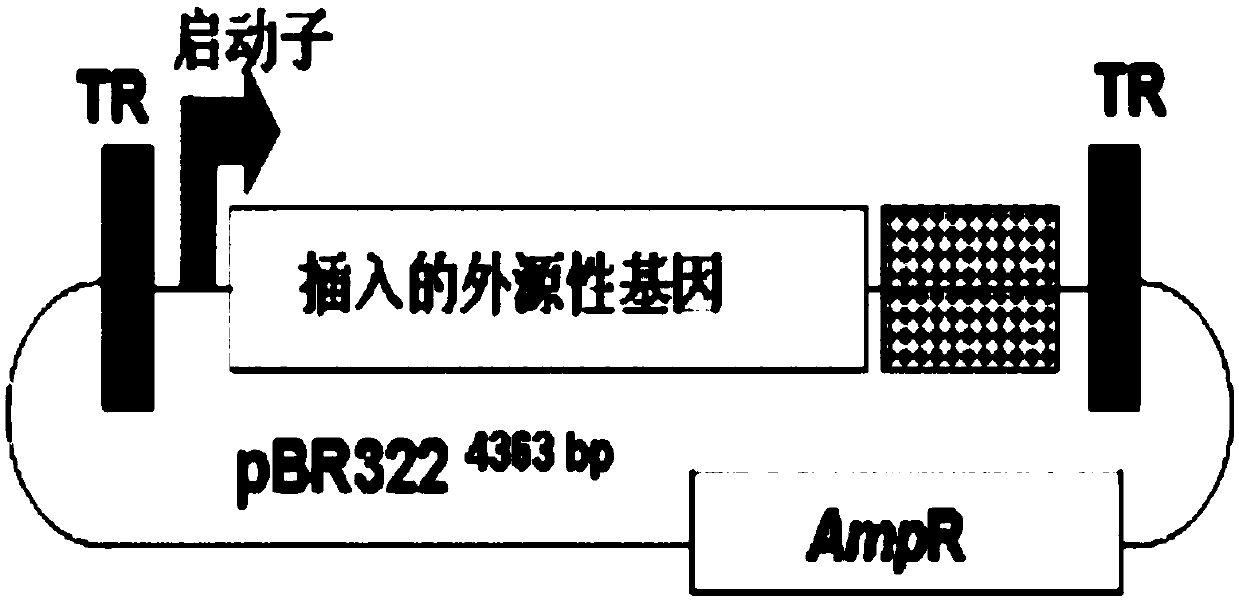
[0074] A. Four kinds of adeno-associated virus (AAV) vectors: pAAV / p5 with AAV p5 promoter, pAAV / CMVp with macrophage virus (CMV) promoter, pAAV / SV40p with SV40 virus early promoter and pAAV / β-actinp with the human β-actin promoter. Known adeno-associated virus vectors have a p5 promoter. In order to increase the transcription level of the target gene, the p5 promoter in the recombinant adeno-associated virus vector can be replaced with a macrophage virus (cytomegalovirus, CMV) promoter, beta actin The promoter and one of the SV40 viral promoters, the four AAV vectors are only different in the promoter, and the rest of the gene structure is exactly the same, that is, it has the complete repeat terminal fragment (TR) sequence at both ends of the AAV type 2, and at both ends of the TR There are 9 nucleotide fragments (CTGCG...
Embodiment 2
[0089] Embodiment 2, preparation of recombinant adeno-associated virus (rAAV) and virus titer determination
[0090] Materials and their sources:
[0091] A. Carrying HPV-16 E7 constructed in Example 1 mm and HPV-16 E7 antigen gene recombinant adeno-associated virus vector (AAV / HPV-16 E7 mm and AAV / HPV-16 E7).
[0092] B. Auxiliary plasmid pHelper containing the Rep gene and Cap gene of AAV: successfully constructed by the inventors of this patent application, Liu Yong et al. (Liu, Y., Santin AD., Mane M., Chiriva-Internati, M., Parham GP ., Ravaggi, A., and Hermonat, P.L. Transduction and Utility of the Granulocyte-Macrophage Colony-Stimulating Factor Gene into Monocytes and Dendritic Cells by Adeno-Associated Virus. Journal of Interferon and Cytokine Research. 20:21–30.2000).
[0093] C. AAV-HEK293 cells containing adenovirus genes (E1, E2A, E4, VAI and VAII genes) integrated in the cell chromosome and expressed: established by Liu Yong, the inventor of this patent applic...
Embodiment 3
[0113] Embodiment 3, recombinant adeno-associated virus AAV / HPV-16 E7 mm Observation on tumorigenicity of primary cervical epithelial cells infected by virus and AAV / HPV-16 E7 virus
[0114] Materials and their sources:
[0115] A. rAAV virus: the recombinant adeno-associated virus (AAV / HPV-16 E7 that embodiment 2 obtains mm virus and AAV / HPV-16E7 virus).
[0116] B. Keratinocyte-SCF cell culture medium: purchased from American Life Technology Company.
[0117] C. Primary cervical epithelial cells: obtained by separating from normal cervical epithelial tissue by conventional methods.
[0118] Tumorigenicity Observation Experiment
[0119] Put the primary cervical epithelial cells into a 10.0cm cell culture dish, immediately add 10mL Keratinocyte-SCF cell culture medium, and culture them in a carbon dioxide incubator at 37°C. After the cells are completely attached to the wall, take out the culture dish, remove 7mL of the culture medium, and then add the recombinant adeno-as...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap