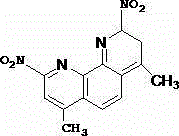
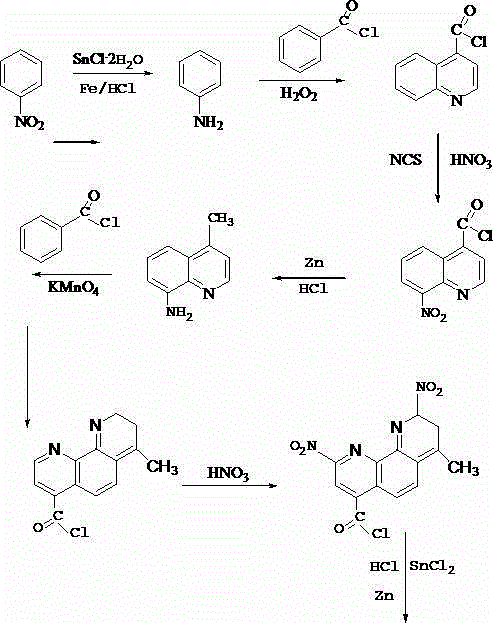
Synthetic method of 2, 9-dinitro-4, 7-dimethyl-1, 10-phenanthroline
A synthetic method, dimethyl technology, applied in the direction of organic chemistry, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0017] First take 150g of nitrobenzene and 30g of SnCl 2 2H 2 After stirring and mixing, put it in the reactor, fill it with hydrogen and add 2g of iron powder, add 500mL of hydrochloric acid solution with a mass fraction of 60% into the reactor, seal it and raise the temperature to 105°C, the pressure is raised to 2MPa, and stir to reduce 4h, cool down to 40°C and filter, heat and melt the filter cake, distill under reduced pressure, collect the fraction at 180°C to obtain crude aniline, then put it into a container, add absolute ethanol to it, stir until the crude aniline is completely dissolved, and then filter , spray-dry the obtained filtrate to obtain aniline; mix and stir the above-prepared aniline and hydrogen peroxide at a mass ratio of 2:1, and after stirring and mixing evenly, slowly add 20 mL of benzoyl chloride dropwise at a temperature of 10°C , stirred for 45min, while adding mass fraction is 95% ethanol, the volume ratio of the amount added to benzoyl chloride...
example 2
[0019]First take 100g of nitrobenzene and 20g of SnCl 2 2H 2 After stirring and mixing, put it in the reactor, fill it with hydrogen and add 1-2g of iron powder, add 300mL of hydrochloric acid solution with a mass fraction of 60% into the reactor, seal it and heat up to 100°C, and the pressure rises to 1.5MPa , stirred and reduced for 2 hours, cooled to 30°C and filtered, heated and melted the filter cake, distilled under reduced pressure, collected the fraction at 120°C to obtain crude aniline, then put it into a container, added absolute ethanol to it, and stirred until the crude aniline was completely After dissolving, filter, and spray-dry the resulting filtrate to obtain aniline; mix and stir the above-prepared aniline and hydrogen peroxide at a mass ratio of 2:1, and after stirring and mixing evenly, slowly add 10 mL of Benzoyl chloride, stirred for 30 minutes, while adding ethanol with a mass fraction of 95%, the volume ratio of the added amount to benzoyl chloride was...
example 3
[0021] First take 120g of nitrobenzene and 25g of SnCl 2 2H 2 After stirring and mixing, put it in the reactor, fill it with hydrogen and add 1g of iron powder, add 400mL of hydrochloric acid solution with a mass fraction of 60% into the reactor, seal and heat up to 101°C, the pressure is raised to 1.7MPa, and stir Restore for 3 hours, cool down to 35°C and filter, heat and melt the filter cake, distill under reduced pressure, collect fractions at 150°C to obtain crude aniline, then put it into a container, add absolute ethanol to it, and stir until the crude aniline is completely dissolved Filtrate, and spray-dry the resulting filtrate to obtain aniline; mix and stir the above-prepared aniline and hydrogen peroxide at a mass ratio of 2:1, and after stirring and mixing evenly, slowly add 15 mL of benzidine dropwise at a temperature of 7°C Acyl chloride, stirred for 40min, while adding ethanol with a mass fraction of 95%, the volume ratio of the added amount to benzoyl chlorid...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
| quality score | aaaaa | aaaaa |
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap