A kind of Fe3O4 magnetic nimodipine liposome and preparation method thereof
A nimodipine lipid and liposome technology, which is applied in the directions of liposome delivery, pharmaceutical formulations, organic active ingredients, etc., can solve problems such as limiting the wide application of magnetic liposomes, reducing the relative space of drugs, and potential safety hazards. , to achieve the effect of high yield, high repeatability and high biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
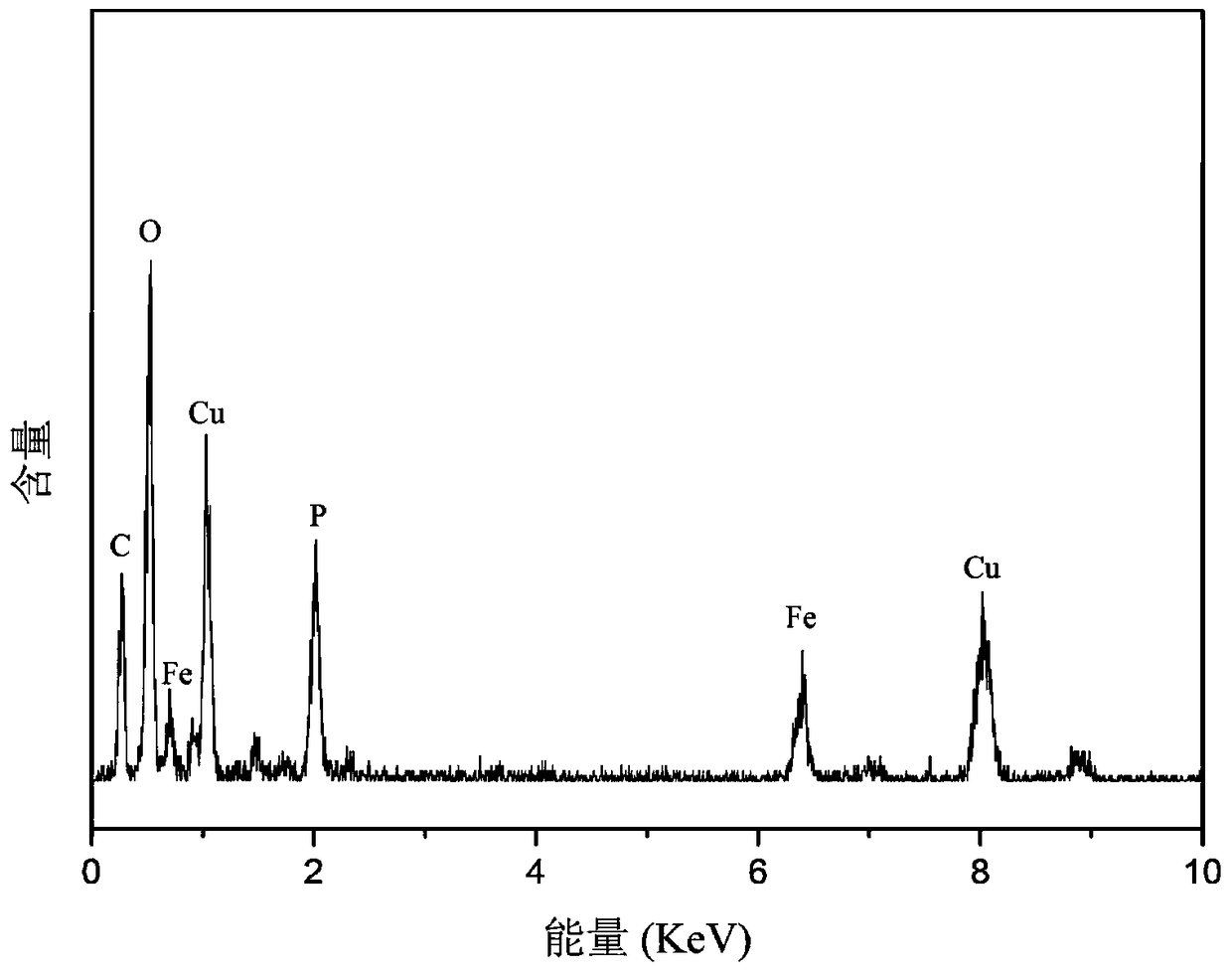
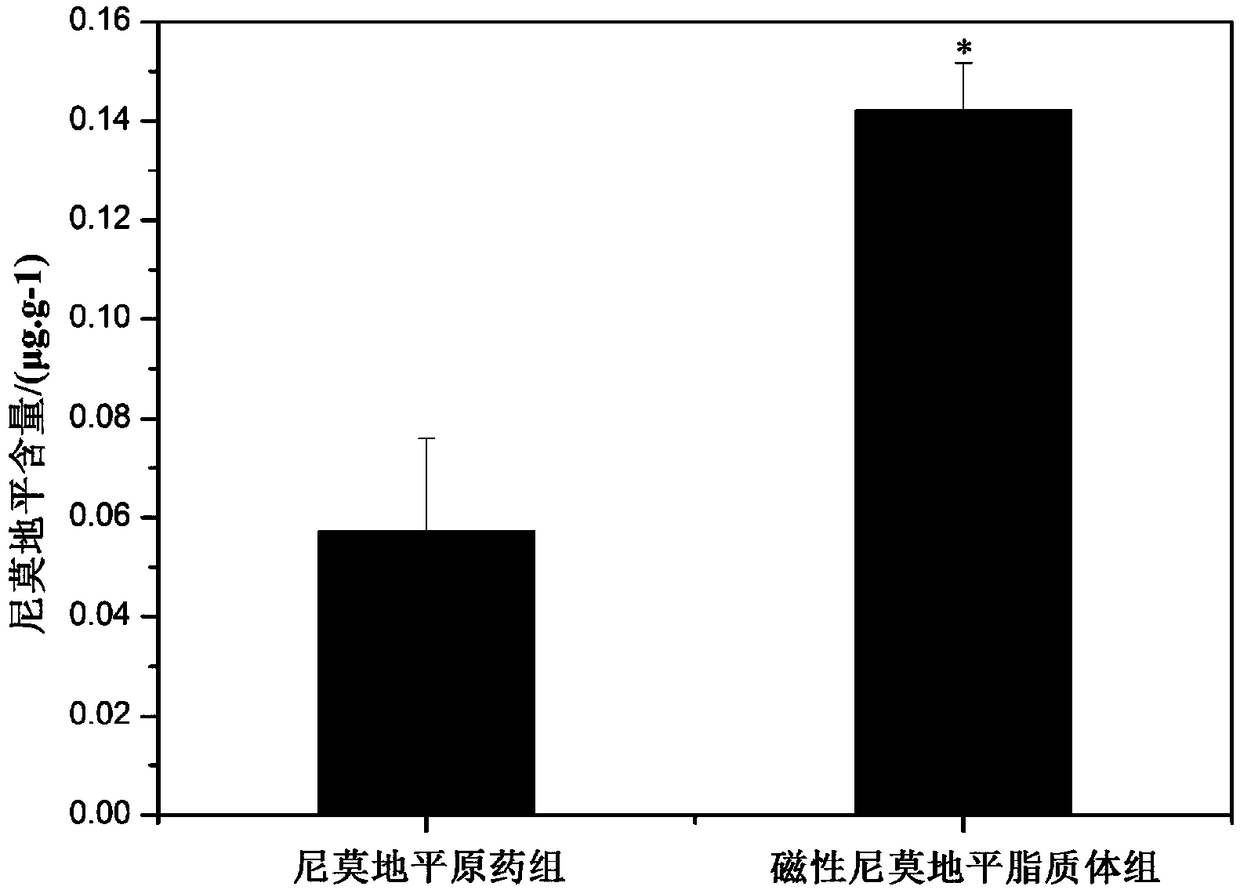
Image
Examples
Embodiment 1
[0022] Take 2 mg of nimodipine (purchased from Nanjing Zelang Biotechnology Co., Ltd.), 40 mg of soybean lecithin (purchased from Shenyang Tianfeng Biopharmaceutical Co., Ltd.) and 4 mg of cholesterol (purchased from Tianjin Damao Chemical Instrument Supply Station) and dissolve with magnetic stirring In 4mL of absolute ethanol, the lipid ethanol solution was obtained. Add the lipid ethanol solution dropwise into 8 mL of phosphate buffer solution (0.1M, pH 6.5), a hydration medium at 40°C, stir slowly with a magnetic stirrer during the dropping process, and remove absolute ethanol by rotary evaporation for 1 hour to obtain Nemo Dipin nanoliposome solution, stored at 4°C for later use;
[0023] FeSO 4 ·7H 2 O and FeCl 3 ·6H 2 O was dissolved in ultrapure water at a molar ratio of 1:2, 0.069 g of polyethylene glycol-2000 was added, and 1 mol / L NaOH solution was added dropwise in a 50°C water bath under ultrasonic until the pH of the solution was 11. Put the solution in a wa...
Embodiment 2
[0027] 2 mg of nimodipine, 50 mg of soybean lecithin and 10 mg of cholesterol were dissolved in 4 mL of absolute ethanol with magnetic stirring to obtain a lipid ethanol solution. Add the lipid ethanol solution dropwise into 10mL phosphate buffer (0.1M, pH 6.5) of the hydration medium at 45°C, stir slowly with a magnetic stirrer during the addition, and stir for 1.5h to remove absolute ethanol to obtain Ni Modipine nanoliposome solution, stored at 4°C for later use;
[0028] FeSO 4 ·7H 2 O and FeCl 3 ·6H 2 O was dissolved in ultrapure water at a molar ratio of 1:2. Add 0.110 g of polyethylene glycol-2000, and add 1 mol / L NaOH solution dropwise under ultrasound in a water bath at 50°C until the pH of the solution is 11. Put the solution in a water bath at 80°C to mature for 15 minutes, magnetically separate, wash with water and ethanol alternately until there is no Cl in the solution - , vacuum the solution at 50mT, freeze-dry at -103°C for 48h, and obtain 0.8g of solid p...
example 3
[0032] 2 mg of nimodipine, 60 mg of soybean lecithin and 16 mg of cholesterol were dissolved in 4 mL of absolute ethanol with magnetic stirring to obtain a lipid ethanol solution. Add the lipid ethanol solution dropwise into 12mL phosphate buffer solution (0.1M, pH6.5, ) of the hydration medium at 50°C, stir slowly with a magnetic stirrer during the dropwise addition, and stir for 2h to remove absolute ethanol to obtain Nemo Dipin nanoliposome solution, stored at 4°C for later use;
[0033] FeSO 4 ·7H 2 O and FeCl 3 ·6H 2 O was dissolved in ultrapure water at a molar ratio of 1:2. Add 0.15 g of polyethylene glycol-2000, and add 1 mol / L NaOH solution dropwise under ultrasound in a 50°C water bath until the pH of the solution is 12. Put the solution in a water bath at 80°C to mature for 15 minutes, magnetically separate, wash with water and ethanol alternately until there is no Cl in the solution - , the solution was vacuum-dried at 50mT, and freeze-dried at -103°C for 48h...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com