Prefusion RSV F proteins and their use
A pre-fusion, protein technology, applied in the pre-fusion RSV F protein and its application field, can solve problems such as unsuccessful
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
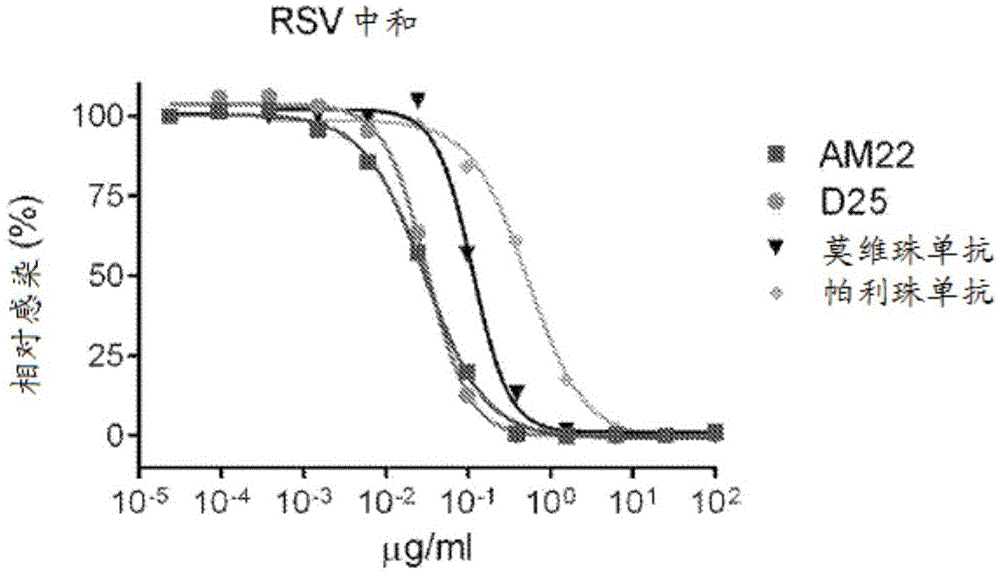
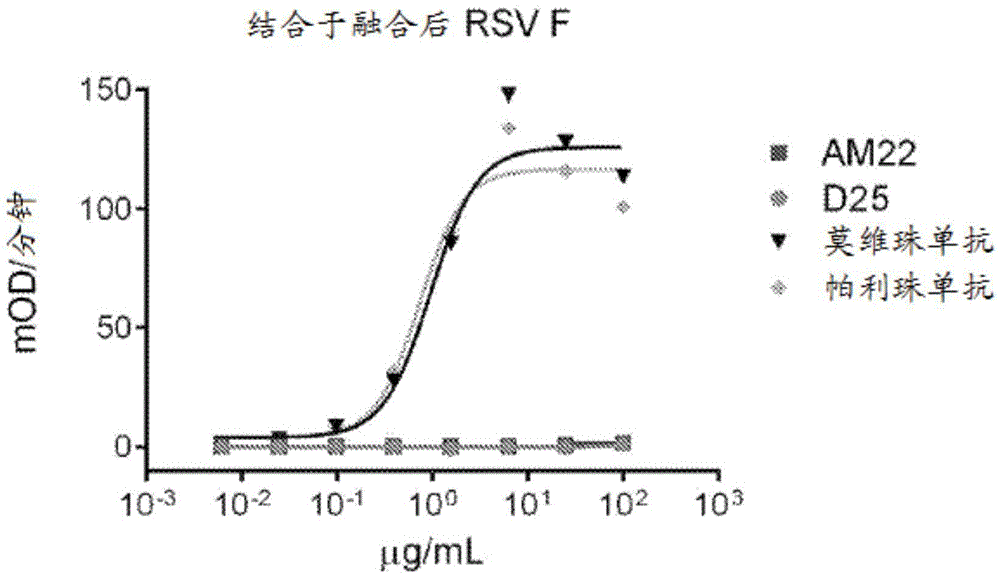
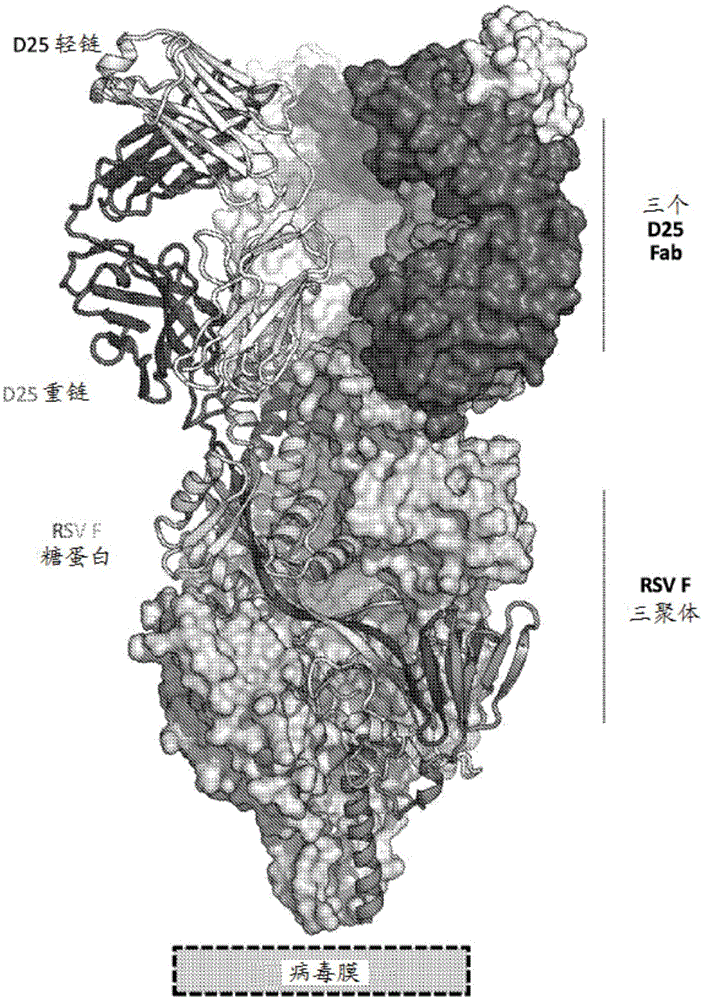
[0858] Structure of the prefusion F trimer of respiratory syncytial virus bound to a human antibody
[0859] The prefusion conformation of the respiratory syncytial virus (RSV) fusion (F) glycoprotein is the target of the majority of RSV neutralizing antibodies in human sera, but its metastability hampers characterization. To overcome this obstacle, a postfusion conformation that does not bind F and is more effective than the prophylactic antibody palivizumab was identified. 10 times larger antibody. The co-crystal structure of one of these antibodies, D25, in complex with the F glycoprotein revealed that D25 locks F in its prefusion state. Comparison of prefusion and postfusion F conformations defines rearrangements required to mediate RSV invasion. The D25-F glycoprotein structure reveals a new vulnerable site, the antigenic site, at the top of the F glycoprotein with prefusion specificity and quaternary properties Structure and antigenic site of RSV F trimer before fus...
Embodiment 2
[0892] Stabilization of RSV F protein
[0893] This example illustrates the design of an exemplary RSV F protein stabilized in a prefusion conformation. Crystal structure of RSV F protein in complex with D25Fab (i.e. in pre-fusion conformation) and post-fusion RSV F protein (for example disclosed in McLellan et al., J. Virol., 85, 7788, 2011, where coordinates are deposited under PDB accession number 3RRR) A comparison of the structures of the membrane revealed a drastic structural rearrangement between the prefusion and postfusion conformations in the membrane-proximal and membrane-distal lobes, providing guidance for the stabilization of the prefusion conformation of RSV F. Based on a comparison of the pre-fusion and post-fusion RSV F structures, there are two regions undergoing large conformational changes, located in the F 1 The N- and C-termini of the subunits. For example, as shown in Figure 2, F 1 Positions 137-216 and 461-513 of the polypeptide undergo structural ...
Embodiment 3
[0933] Membrane-proximal lobe that stabilizes PreF antigen
[0934] As discussed above, the crystal structure of the RSV F protein in complex with D25Fab (i.e., in a prefusion conformation) and the postfusion RSV F protein (for example, are disclosed in McLellan et al., J. Virol., 85, 7788, 2011, where coordinates are in PDB A comparison of the structure of accession number 3RRR) reveals a drastic structural rearrangement between the prefusion and postfusion conformations in the distal lobe of the membrane. Based on a comparison of the pre-fusion and post-fusion RSV F structures, there are two regions undergoing large conformational changes, located in the F 1 The N- and C-termini of the subunits. For example, as shown in Figure 2, F 1 Positions 137-216 and 461-513 of the polypeptide undergo structural rearrangements between the prefusion and postfusion F protein conformations, while the F 1 Positions 271-460 of the polypeptide remained relatively unchanged. This example...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com