Novel formulation of cilostazol, a quinolinone-derivative used for alleviating the symptom of intermittent claudication in patients with peripheral, vascular disease
A technology of cilostazol and composition, applied in the field of pharmaceutical preparations, capable of solving problems such as adverse reactions of cilostazol
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1-4
[0045] Examples 1-4: Cilostazol Extended Release Lozenges and Their In Vitro Dissolution Profiles
[0046] PMR Examples 1-4, each containing 100 mg of non-particulate cilostazol, were prepared following Workflow Mode 1 described in Table 1 from the ingredients shown in Table 2 below.
[0047] Table 2. Compositions for PMR Examples 1-4
[0048]
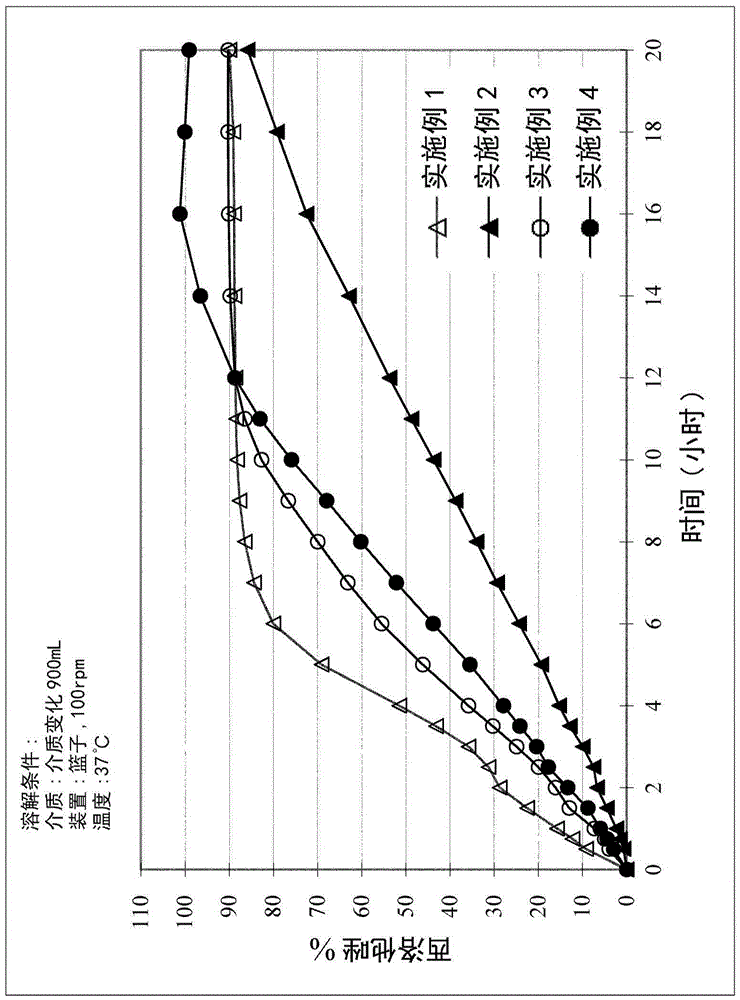
[0049] A study was performed to evaluate the in vitro dissolution profiles of Examples 1-4. The study was performed according to the procedures described in the United States Pharmacopeia (USP36, 2031). More specifically, Examples 1-4 were each placed in a dissolution medium at a temperature of about 37°C, and the dissolution medium was stirred at a speed of about 50 or 100 rpm. The concentration of cilostazol in the dissolution medium was measured at different time periods. The results are shown in Table 3 below and figure 1 middle.
[0050] Table 3. In Vitro Dissolution Profiles of PMR Examples 1-4
[0051]
[0052] The r...
example 5-7
[0053] Examples 5-7: Cilostazol Extended Release Lozenges and Their In Vitro Dissolution Profiles
[0054] PMR Examples 5-7, each containing 100 mg of particulate cilostazol, were prepared following Workflow Mode 1 described in Table 1 from the ingredients shown in Table 4 below. It is noted that the particulate cilostazol used in Examples 5-7 had a D(0.9) of 5.1-75.2 μm compared to Examples 1-4.
[0055] Table 4. Compositions for PMR Examples 5-7
[0056]
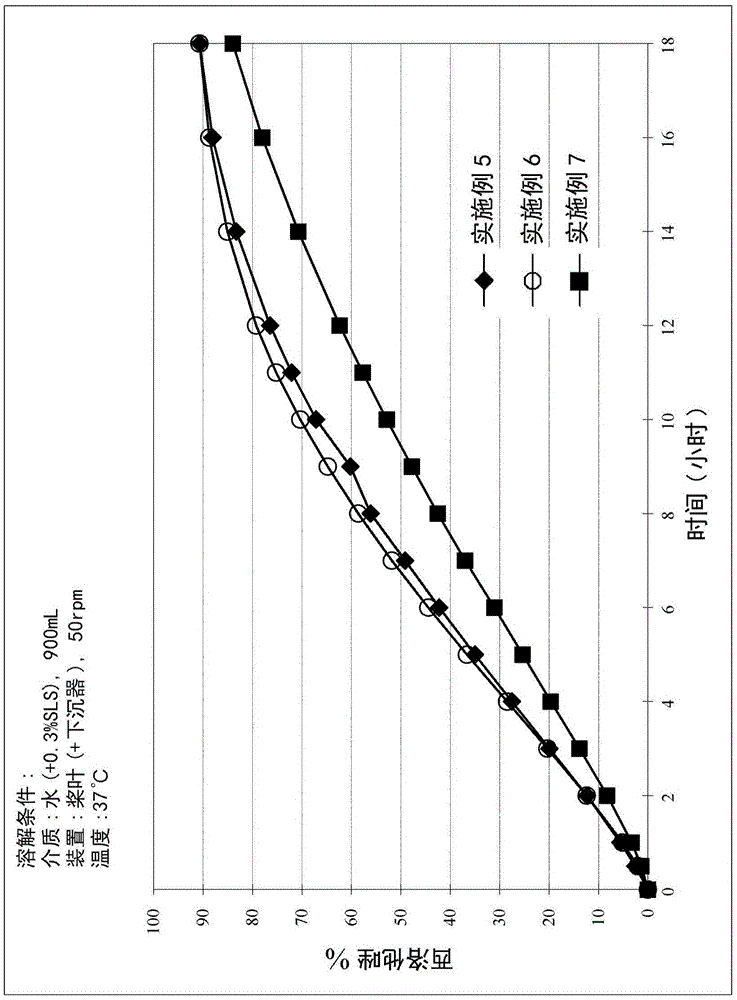
[0057] Studies were performed to evaluate the in vitro dissolution profiles of Examples 5-7. The study was performed according to the procedure described above. The results are shown in Table 5 below and figure 2 middle.
[0058] Table 5. In Vitro Dissolution Profiles of PMR Examples 5-7
[0059]
[0060]
[0061] These results show that, compared to Examples 1-4, Examples 5-7 prepared with particulate cilostazol exhibited a more consistent zero-degree release profile in the dissolution medium. See figure ...
example 8-10
[0062] Examples 8-10: Cilostazol Extended Release Lozenges and Their In Vitro Dissolution Profiles
[0063] PMR Examples 8-10, each containing 100 mg of microparticulate cilostazol, were prepared following Workflow Mode 2 described in Table 1 from the ingredients shown in Table 6 below.
[0064] Table 6. Compositions for PMR Examples 8-10
[0065]
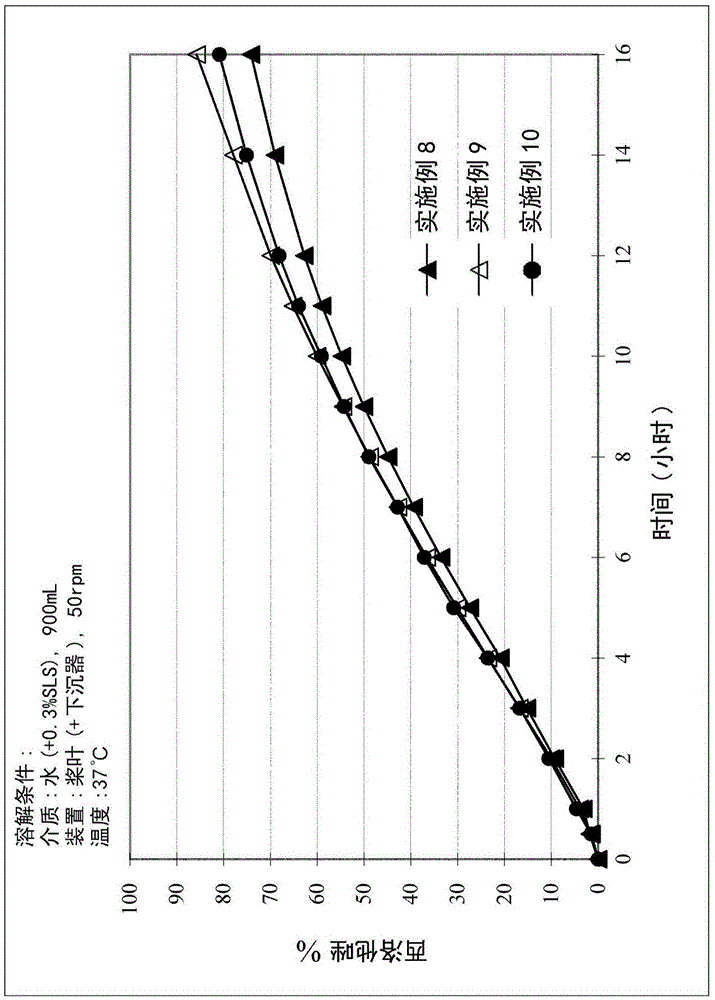
[0066] A study was performed to evaluate the in vitro dissolution profiles of Examples 8-10. The study was performed according to the procedure described above. The results are shown in Table 7 below and image 3 middle.
[0067] Table 7. In Vitro Dissolution Profiles of PMR Examples 8-10
[0068]
[0069] These results show that varying amounts of povidone product used in preparation did not affect the in vitro dissolution profile of PMR lozenges.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com