Therapeutic use of VEGF-C and CCBE1
A VEGF-C, CCBE1 technology, applied in gene therapy, medical preparations containing active ingredients, chemical instruments and methods, etc., can solve the problem of no cure for lymphedema
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
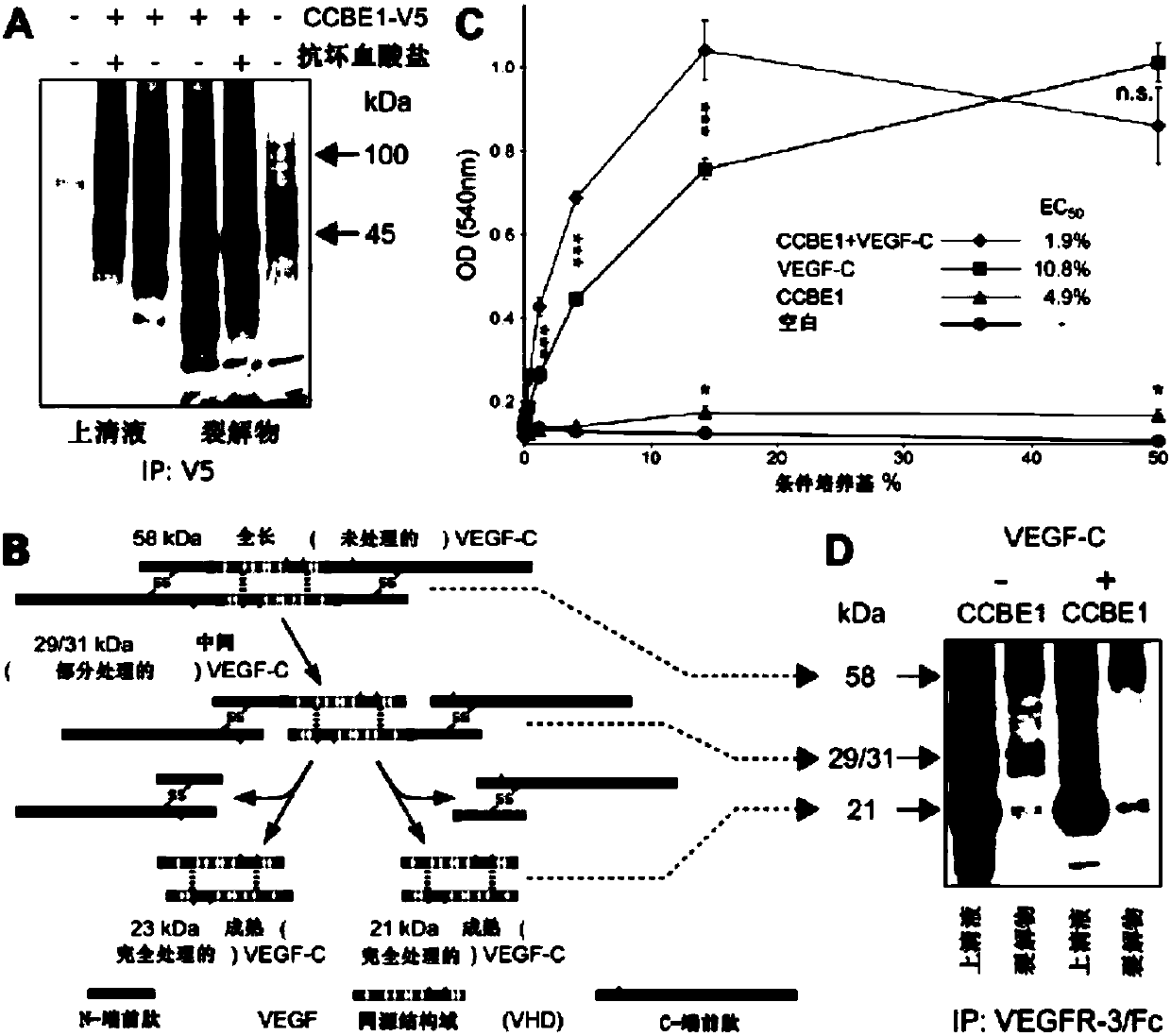
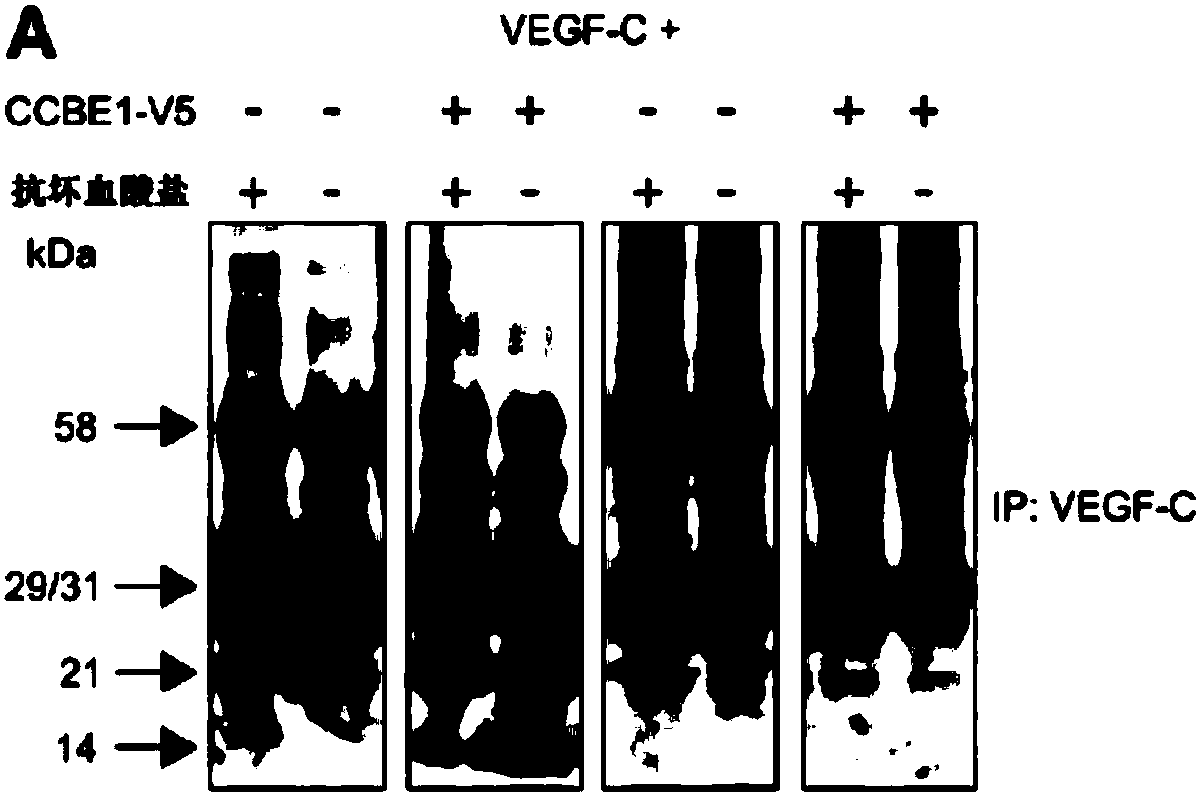
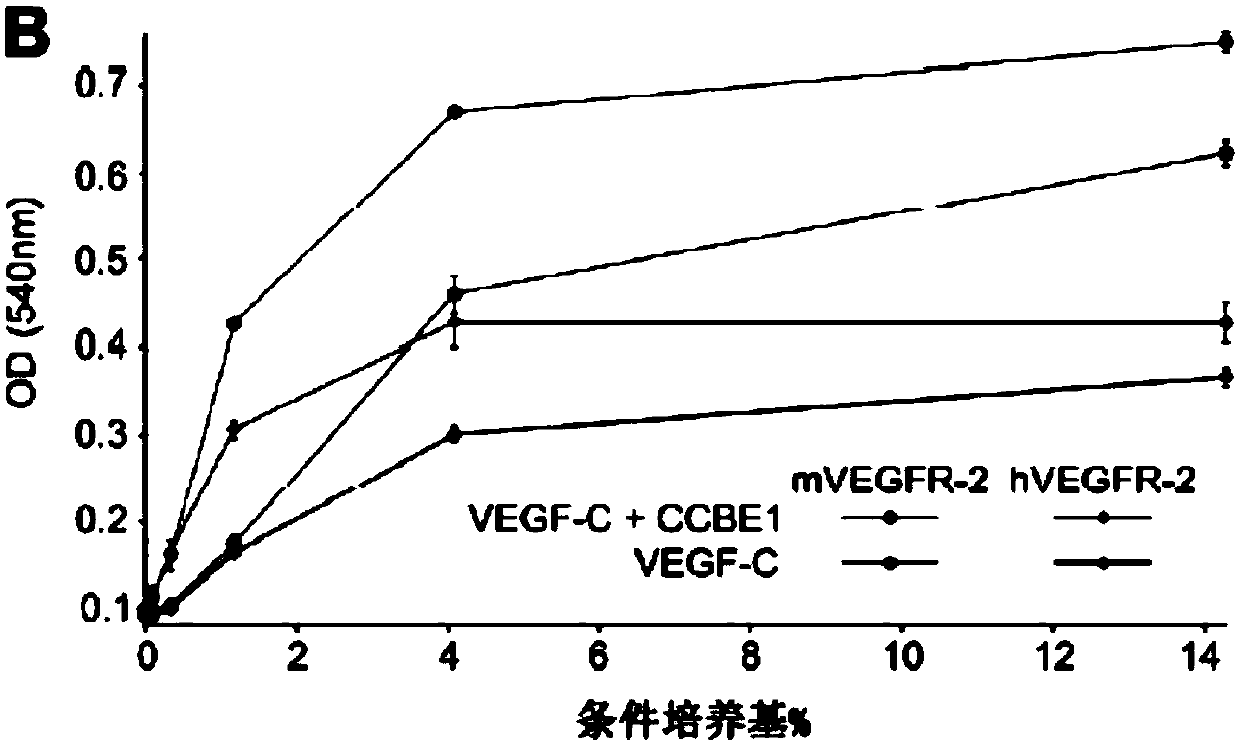
[0068] Materials and methods
[0069] clone. Genes expressed by recombinant adeno-associated virus (rAAV9) vectors were cloned into the psubCAG-WPRE plasmid (Paterna et al., 2000, GeneTherapy (Gene Therapy) 7:1304-1311), which is a derivative of psubCMV-WPRE in which the CMV promoter has been replaced by a composite CAG promoter consisting of the chicken β-actin promoter, cytomegalovirus enhancer and β-actin intron (Okabe et al., 1997 , FEBS Letters 407:313-319). Cloning of full-length mVEGF-C, ΔNΔC-mVEGF-C and HAS into an AAV-vector (psubCAG-WPRE) has been described earlier (Anisimov et al., 2009, supra). mCCBE1 fused to the V5 marker (mCCBE1-V5) was cloned as follows: Part of the coding DNA sequence of CCBE1 (CDS; GenBank #BC152322, image clone ID40140631) was cloned as a SacI / XbaI fragment into pVK1 (pUC19-derived vector ( Anisimov et al., 2007, Molecular Breeding 19:241-253)). Brown adipose tissue mRNA was amplified with primers 5'-GCCGCTAGCGCCACCATGGTGCCGCCGCCT-3' (SE...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com