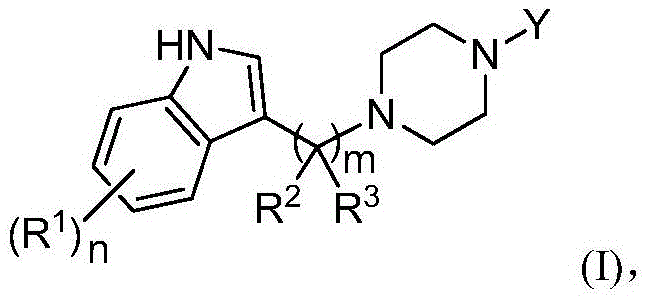
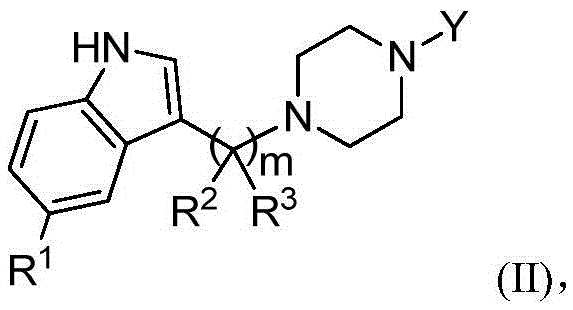
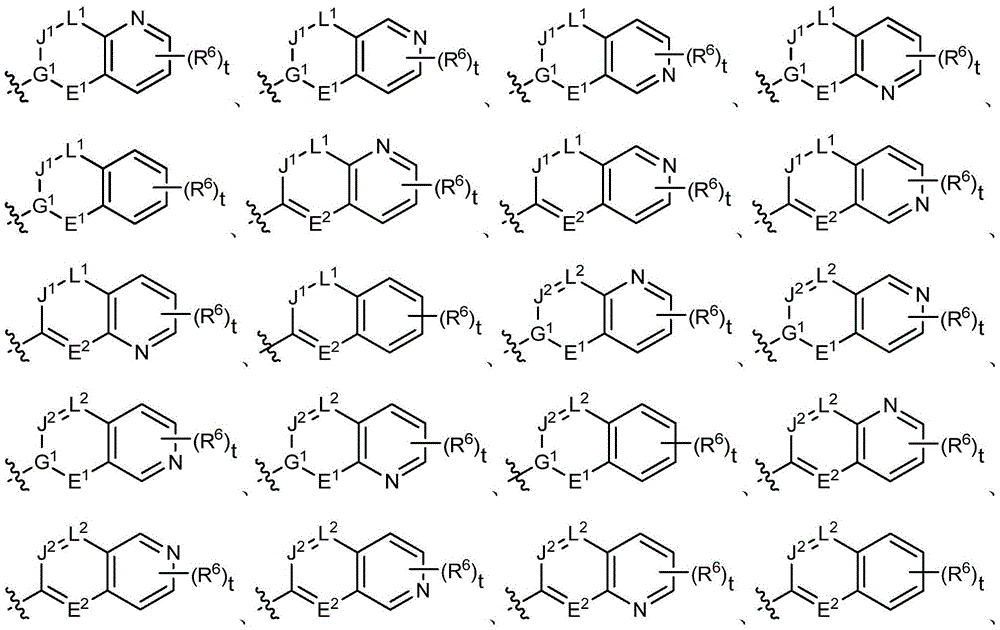
Substituted piperazine compounds, application method and applications thereof
A compound, nitrogen oxide technology, applied in the field of medicine, can solve the problems of reducing the concentration of serotonin and reducing the release of serotonin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 19-32
[0373] Synthesis of target compound in Table A embodiment 19-32 of the present invention
[0374]
[0375]
[0376]
[0377] biological test
Embodiment A
[0378] Example A: Compounds in rat synaptosomes on [ 3 Analysis of the inhibitory effect of H]5-HT uptake
[0379] experiment method
[0380] At 37°C, add to buffer (106.2mMNaCl, 4.5mMKCl, 2.25mMMgSO 4 ,1.08mMNaH 2 PO 4 ,22.5mM NaHCO 3 , 9.9 mM glucose, 9 μM GTA and 45 μM ascorbic acid (pH 7.4)), synaptosomes (150 μg) with 0.1 μCi[ 3 H] In the mixed system formed by 5-hydroxytryptamine, add test compound or positive drug or negative control, and incubate for 15 minutes.
[0381] Imipramine is used as a standard positive compound for inhibiting the uptake of serotonin. Add 10 μM imipramine to the same mixed system as above to block the uptake of serotonin, and incubate at 4°C for 15 minutes to measure the activity value of the basic control . Through experiments, the uptake inhibition value of different concentrations of imipramine on rat brain synaptosomes was tested, and an inhibition curve was made.
[0382] After incubation, the sample was quickly filtered t...
Embodiment B
[0388] Example B: h5-HT 1A Receptor Binding Affinity Assay
[0389] experiment method
[0390] Homogenate (36μg protein) to human HEK-293 cell membrane at 22°C, 0.3nM[ 3 H]8-OH-DPAT (Perkin-Elmer) and buffer (50mM Tris-HCl (pH7.4), 10mMMgSO 4 , 0.5mM EDTA, 2μg / mlaprotinine) into the mixed system formed, with or without adding the test compound, and incubated for 60 minutes.
[0391] The standard reference compound is 8-OH-DPAT, and 10 μM 8-OH-DPAT is added to the mixed system under the above conditions to measure the non-specific binding value. The data of series concentrations of 8-OH-DPAT were tested in different experiments to obtain the competition curve.
[0392] After incubation, the sample was quickly filtered through a glass fiber filter (GF / B, Packard) pre-soaked with 0.3% PEI using a 96-sample cell harvester (Unifilter, Packard) under vacuum, and rinsed repeatedly with ice-cold 50 mM Tris-HCl. several times. Filters were dried and residual radioactivity...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com