A tetravalent manganese ion-doped ammonium salt red light material and preparation method thereof
A technology of ion doping and tetravalent manganese, applied in luminescent materials, chemical instruments and methods, sustainable buildings, etc., can solve the problems of no commercial sales, complicated preparation process, and pollution of target products, and achieve low cost and synthetic design The effect of low requirements and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
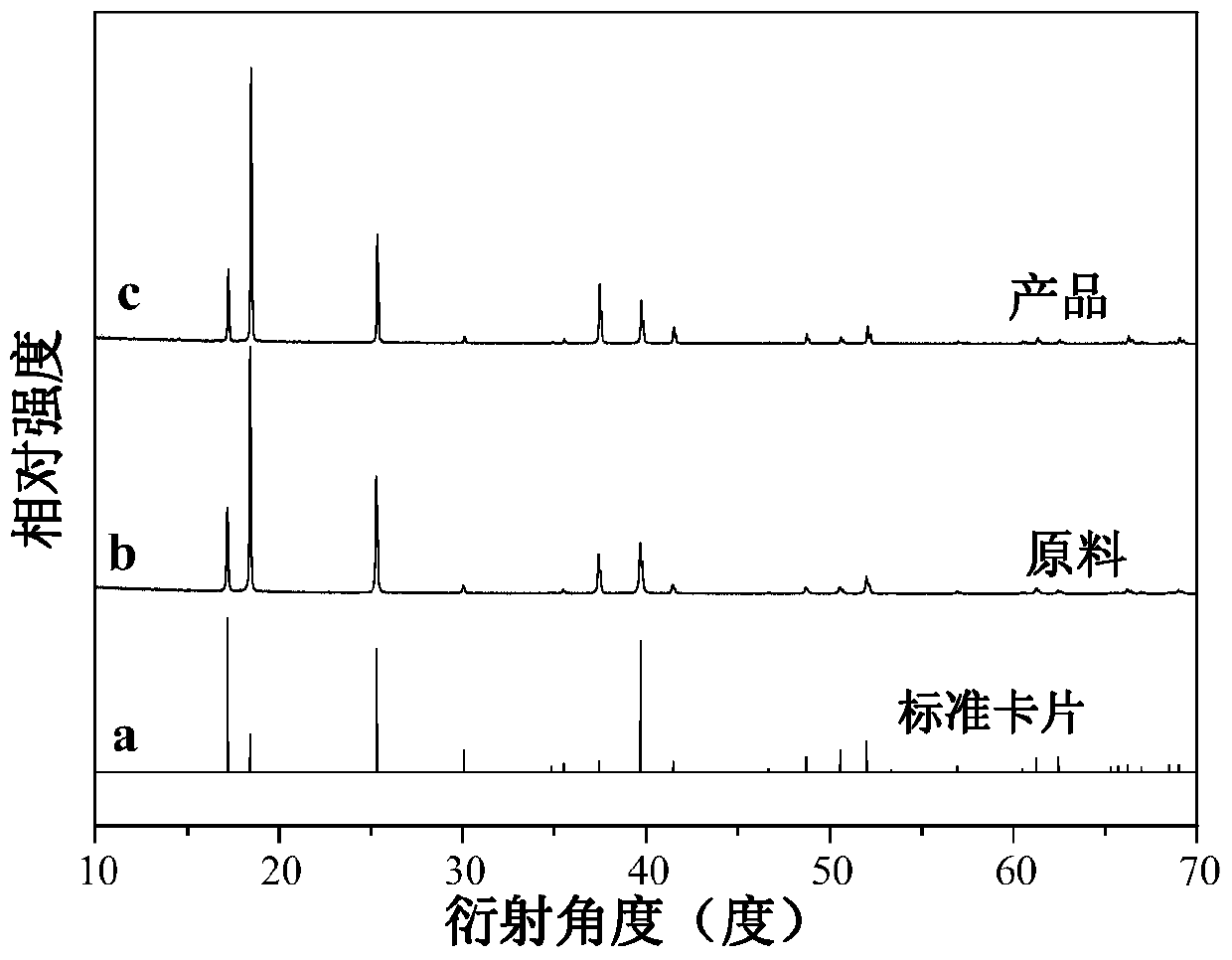
[0029] Prepare KMnO with a concentration of 0.05mol / L 4 aqueous solution as raw material. Weigh 2.5g solid (NH 4 ) 2 TiF 6 Place in a plastic beaker, add KMnO successively dropwise 4 Aqueous solution, HF aqueous solution, and deionized water are added to make KMnO in the reaction system 4 The molar concentration is 5×10 ‐4 mol / L, the mass concentration of HF was 16%, stirred at room temperature for 8 hours, filtered with suction, and dried naturally. Its XRD (BrukerD8Advance X-ray diffractometer detects) such as figure 1 As shown, XRD shows that the product is about pure (NH 4 ) 2 TiF 6 Mutually.
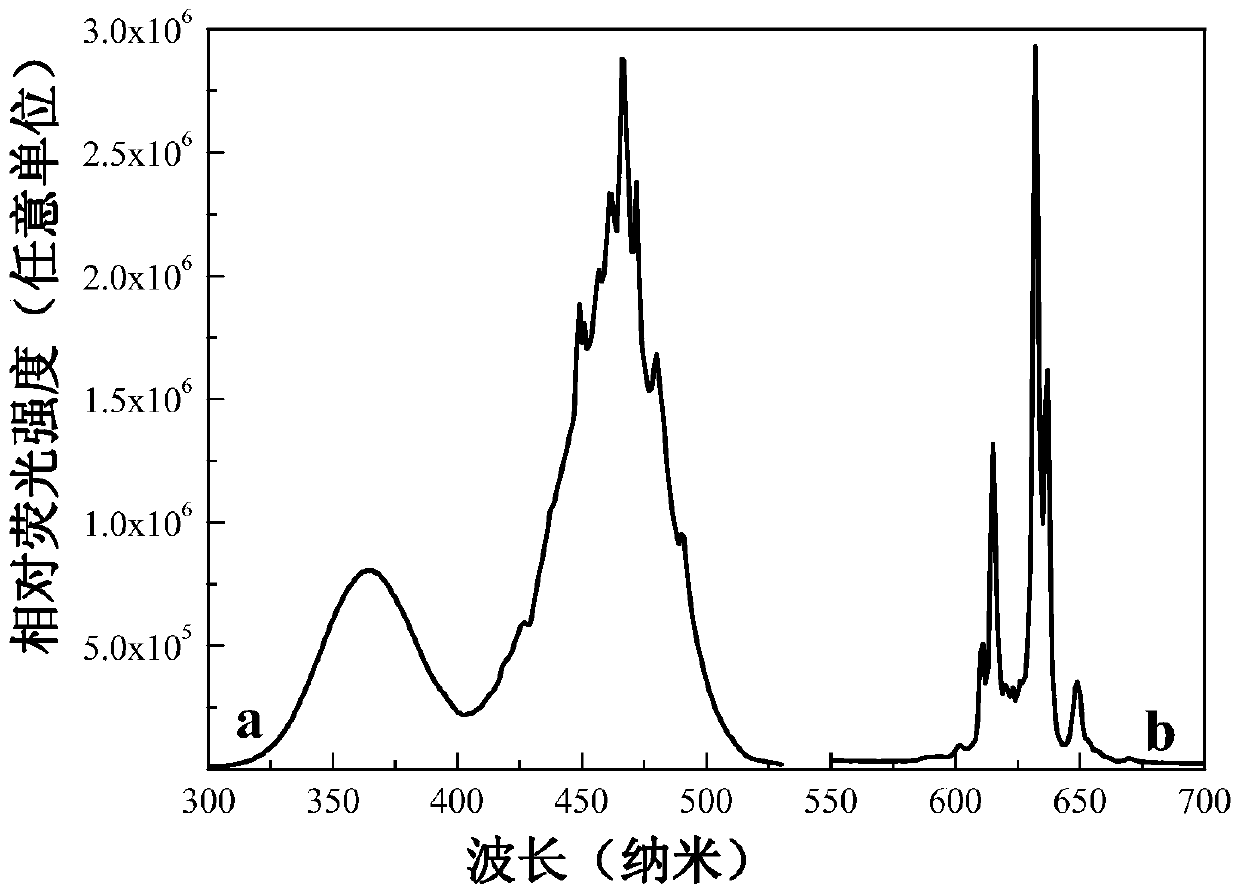
[0030] After testing, the product obtained in this embodiment is light yellow crystal under natural light, and bright red light is found under ultraviolet light. Using a Fluoromax‐4 fluorescence spectrometer (HORIBA Jobin Yvon Inc.), the luminescent properties of the product were detected at room temperature, such as figure 2 As shown, the excitation spectrum of the mat...
Embodiment 2
[0034] Prepare KMnO with a concentration of 0.05mol / L 4 aqueous solution as raw material. Weigh 2.0g solid (NH 4 ) 2 TiF 6 Place in a plastic beaker, add KMnO successively dropwise 4 Aqueous solution, HF aqueous solution, and deionized water are added to make KMnO in the reaction system 4 The molar concentration is 1×10 ‐3 mol / L, the mass concentration of HF was 4%, stirred at room temperature for 8 hours, filtered with suction, and dried naturally. Pale yellow crystals were obtained. The product glows red under a UV light. The product is a toner material, the XRD pattern, product photo, excitation spectrum and emission spectrum of the white powder material are in accordance with figure 1 , 2 basically the same.
Embodiment 3
[0036] Prepare KMnO with a concentration of 0.05mol / L 4 aqueous solution as raw material. Weigh 5.0g solid (NH 4 ) 2 TiF 6 Place in a plastic beaker, add KMnO successively dropwise 4 Aqueous solution, HF aqueous solution, and deionized water are added to make KMnO in the reaction system 4 The molar concentration is 1×10 ‐4 mol / L, the mass concentration of HF was 20%, stirred at room temperature for 12 hours, filtered with suction, and dried naturally. Pale yellow crystals were obtained. The product glows red under a UV light. The product is a toner material, the XRD pattern, product photo, excitation spectrum and emission spectrum of the white powder material are in accordance with figure 1 , 2 basically the same.
PUM
| Property | Measurement | Unit |
|---|---|---|
| quantum efficiency | aaaaa | aaaaa |
| spectroscopy | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com