Use of a pcsk9 inhibitor to treat hyperlipidemia
A technology for inhibitors and hypercholesterolemia, applied in the field of PCSK9 inhibitors to treat patients with hyperlipidemia who are not receiving statin therapy, and can solve problems such as statin intolerance, non-compliance, and poor control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0116] Production of Human Antibodies
[0117] Methods for producing human antibodies in transgenic mice are known in the art. Any such known method may be used in the context of the present invention to prepare human antibodies that specifically bind human PCSK9.
[0118] Use VELOCIMMUNE TM technology (see, eg, US6,596,541, Regeneron Pharmaceuticals) or any other known technique for generating monoclonal antibodies, initially isolate a high affinity chimeric antibody against PCSK9 having human variable regions and mouse constant regions. The technique involves generating a transgenic mouse having a genome comprising human heavy and light chain variable regions operably linked to endogenous mouse constant region loci such that the mouse produces genes comprising human variable regions in response to antigenic stimuli. and mouse constant region antibodies. The DNA encoding the variable regions of the heavy and light chains of the antibody is isolated and operably linked to ...
Embodiment 1
[0168] Example 1. Generation of human antibodies against human PCSK9
[0169] Human anti-PCSK9 antibodies were generated as described in US Patent No. 8,062,640. An exemplary PCSK9 inhibitor used in the following examples is a human anti-PCSK9 antibody designated "mAb316P", also known as "REGN727" or "alirocumab". mAb316P has the following amino acid sequence characteristics: a heavy chain comprising SEQ ID NO:5 and a light chain comprising SEQ ID NO:9; a heavy chain variable region (HCVR) comprising SEQ ID NO:1 and a light chain variable domain (LCVR) comprising SEQ ID NO:6 ); heavy chain complementarity determining region 1 (HCDR1) comprising SEQ ID NO:2, HCDR2 comprising SEQ ID NO:3, HCDR3 comprising SEQ ID NO:4, light chain complementarity determining region 1 (LCDR1) comprising SEQ ID NO:7, comprising SEQ ID NO:8 LCDR2 and LCDR3 comprising SEQ ID NO:10.
Embodiment 2
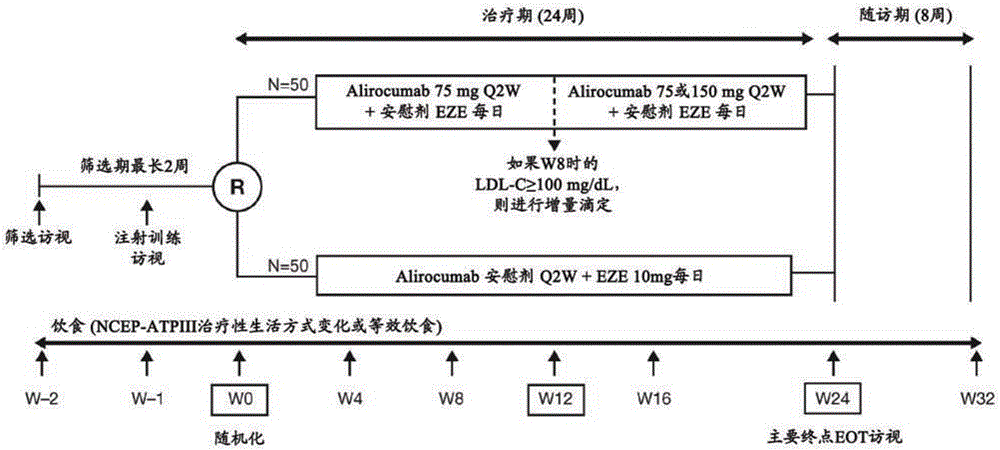
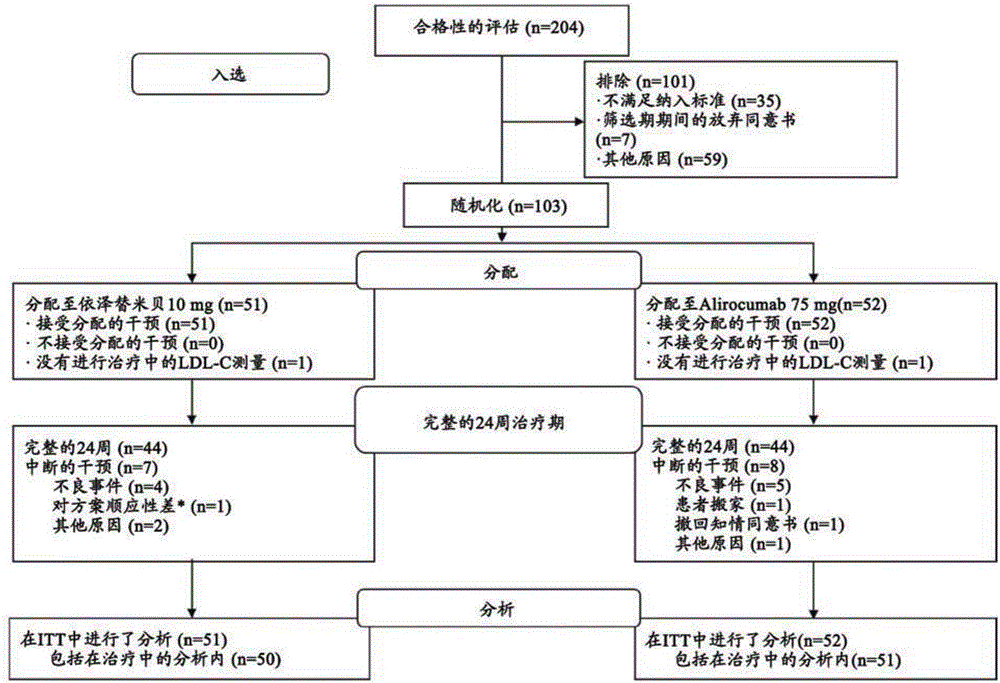
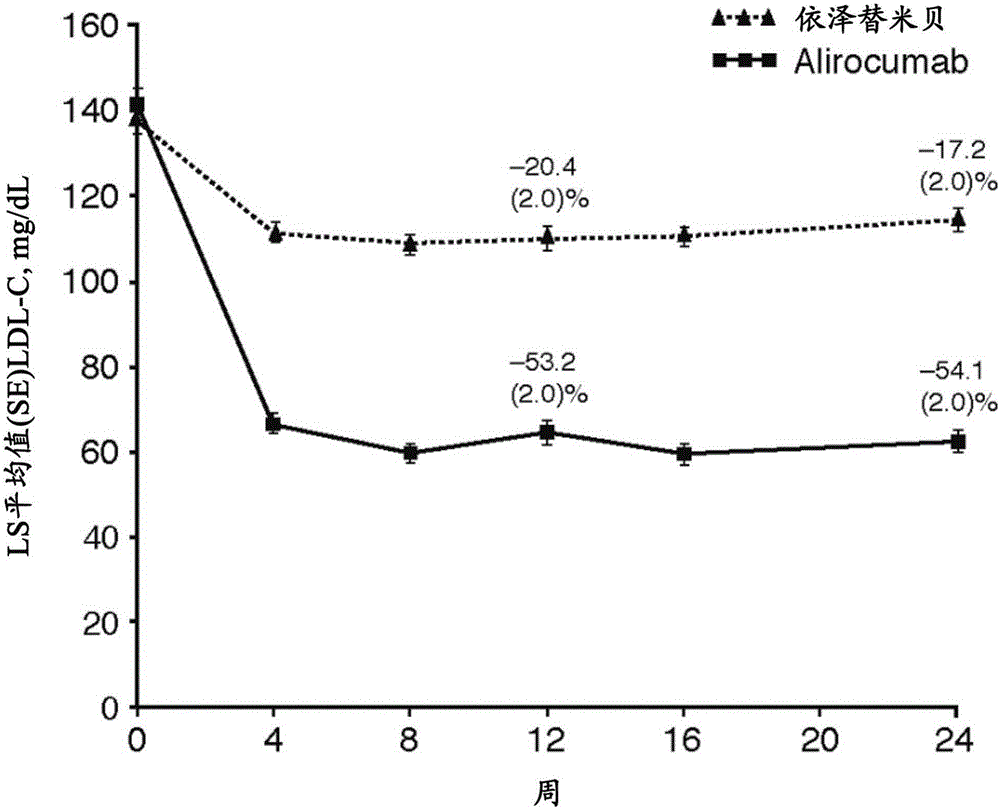
[0170] Example 2: Monotherapy with an anti-PCSK9 antibody ("mAb316P") versus ezetimibe in patients with hypercholesterolemia: results of a 24-week, double-blind, randomized phase 3 clinical trial
[0171] background
[0172] Hypercholesterolemia, especially increased levels of low-density lipoprotein cholesterol (LDL-C), constitutes a major risk for the development of atherosclerosis and CHD, the leading causes of death and disability in the Western world . LDL-C has been identified as a major target of cholesterol-lowering therapy and is recognized as a valid surrogate endpoint. Multiple studies have demonstrated that lowering LDL-C levels reduces CHD risk primarily through 3-hydroxy-3-methyl-glutaryl-CoA reductase (HMGCoA) inhibition with statins, with a relationship between LDL-C levels and CHD events There is a strong direct relationship; for every 1 mmol / L (approximately 40 mg / dL) reduction in LDL-C, there is a 22% reduction in cardiovascular disease (CVD) mortality and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com