Medicine for treating gynecologic inflammation and preparation method thereof
A technology for gynecological inflammation and medicine, which is applied in the field of medicine for treating gynecological inflammation and its preparation, and can solve problems such as poor stability, protein inactivation or denaturation, and ineffective viral infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
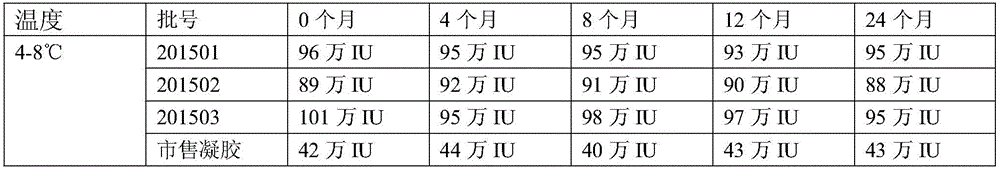
[0055] A medicine for treating gynecological inflammation, the medicine comprises freeze-dried powder solution and freeze-dried powder.
[0056] Prescription of lyophilized powder solution: carboxymethyl chitosan 60g, nano silver 3g, iodophor 6g and purified water 3L.
[0057] Production process of freeze-dried powder solution: Dissolve carboxymethyl chitosan, nano-silver, and iodophor in 3L of purified water. After the dissolution is complete, filter with a 0.22 micron sterile filter, fill, press rubber stopper and aluminum cover, That is, the lyophilized powder solution is obtained.
[0058] Freeze-dried powder prescription: recombinant human interferon 1,000,000,000 units, mannitol 50g and purified water 1L.
[0059] Freeze-dried powder production process: Weigh 1,000,000,000 units of recombinant human interferon and 50g of mannitol in proportion, dissolve them in 1L of purified water; after the dissolution is complete, filter with a 0.22 micron sterile filter, fill 1ml in...
Embodiment 2
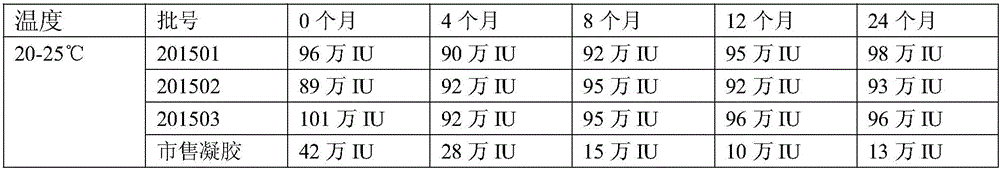
[0064] A medicine for treating gynecological inflammation, the medicine comprises freeze-dried powder solution and freeze-dried powder.
[0065] The prescription of freeze-dried powder solution: carboxymethyl chitosan 70g, nano-silver 6g, iodophor 9g and purified water 3L.
[0066] The production process of freeze-dried powder solution: take carboxymethyl chitosan, nano-silver, and iodophor dissolved in purified water, after the dissolution is complete, filter with a 0.22 micron sterile filter, fill, press rubber plug and aluminum cover, that is Obtain freeze-dried powder solution.
[0067] Freeze-dried powder prescription: recombinant human interferon 1,000,000 units, mannitol 60g and purified water 1L.
[0068] Freeze-dried powder production process: Weigh 1,000,000,000 units of recombinant human interferon and 50g of mannitol in proportion, dissolve them in 1L of purified water; after the dissolution is complete, filter with a 0.22 micron sterile filter, fill 1ml into a 4....
Embodiment 3
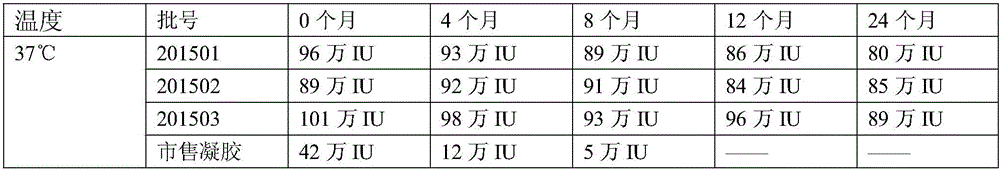
[0071] A medicine for treating gynecological inflammation, the medicine comprises freeze-dried powder solution and freeze-dried powder.
[0072] The prescription of the lyophilized powder solution: carboxymethyl chitosan 50g, nano silver 1g, iodophor 3g and purified water 3L.
[0073] The production process of freeze-dried powder solution: take carboxymethyl chitosan, nano-silver, and iodophor dissolved in purified water, after the dissolution is complete, filter with a 0.22 micron sterile filter, fill, press rubber plug and aluminum cover, that is Obtain freeze-dried powder solution.
[0074] Freeze-dried powder prescription: recombinant human interferon 1,000,000 units, mannitol 40g and purified water 1L.
[0075] Freeze-dried powder production process: Weigh 1,000,000,000 units of recombinant human interferon and 50g of mannitol in proportion, dissolve them in 1L of purified water; after the dissolution is complete, filter with a 0.22 micron sterile filter, fill 1ml into a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com