Compretidine drug precursor, pharmaceutical preparation and preparation method
A technology of compretidine and drugs, which is applied in the field of drug synthesis, can solve the problems of rapid release of drugs, achieve the effect of prolonging the circulation, good clinical application prospects and application value, and improving the effect of anti-angiogenesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
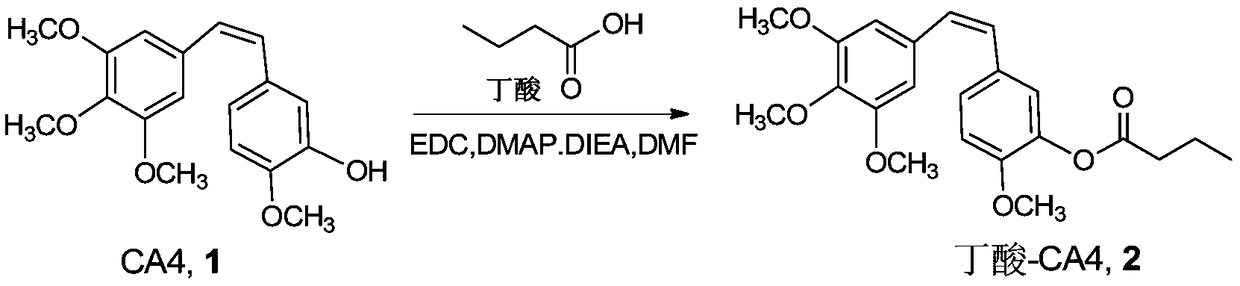
[0044] Butyric acid (51.7 μL, 0.57 mmol), CA4 (compound 1, 180 mg, 0.57 mmol), EDC·HCl (163 mg, 0.855 mmol), DMAP (76.6 mg, 0.627 mmol), DIEA (188.9 μL, 1.14 mmol) were dissolved In 4ml of DCM, stirred overnight at room temperature, added ethyl acetate, respectively with 5% citric acid, saturated NaHCO 3 , saturated brine to wash the organic phase, the organic phase was dried with anhydrous sodium sulfate, filtered, and the solvent was removed under reduced pressure after collecting the filtrate; the solid was separated and purified by column chromatography (ethyl acetate:n-hexane=1:3), to obtain the target The product butyric acid-CA4 conjugate compound 2 (280 mg, yield 93%). Synthetic route such as figure 1 shown.
[0045] The 1H NMR nuclear magnetic data and mass spectral data of product butyric acid-CA are as follows:
[0046] 1 H NMR (400MHz, CDCl 3 ):δ1.00-1.04(t,3H),1.73-1.78(m,2H),2.49-2.53(t,2H),3.71(s,6H),3.80(s,3H),3.83(s,3H ),6.45-6.45(d,2H,J=1.2),6.51(s,2H),...
Embodiment 2
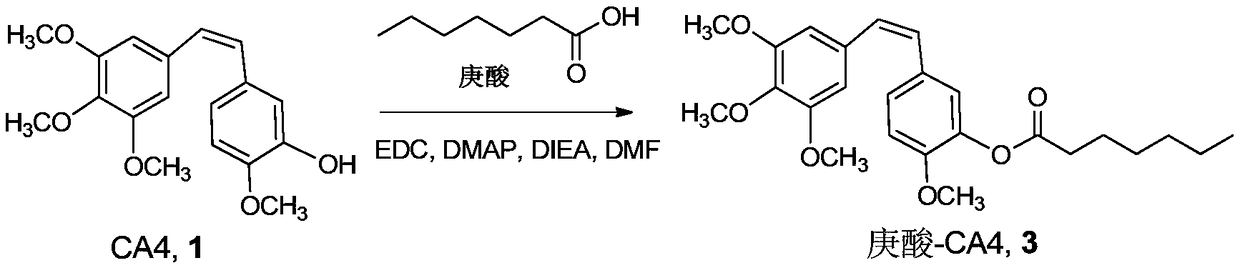
[0049] Heptanoic acid (80.8 μL, 0.57 mmol), CA4 (180 mg, 0.57 mmol), EDC·HCl (163 mg, 0.855 mmol), DMAP (76.6 mg, 0.627 mmol), DIEA (188.9 μL, 1.14 mmol) were dissolved in 4 mL of DCM , stirred overnight at room temperature, added ethyl acetate, and successively washed with 5% citric acid, saturated NaHCO 3 , saturated brine to wash the organic phase, the organic phase was dried with anhydrous sodium sulfate, filtered, and the solvent was removed under reduced pressure after collecting the filtrate; the solid was separated and purified by column chromatography (ethyl acetate:n-hexane=1:3), to obtain the target The product heptanoic acid-CA4 conjugate compound 3 (260 mg, yield 85%). Synthetic route such as figure 2 shown.
[0050] 1 H NMR (400MHz, CDCl 3 ):δ0.88-0.91(t,3H),1.26-1.41(m,6H),1.70-1.74(t,2H),2.51-2.54(t,2H),3.71(s,6H),3.80(s ,3H),3.84(s,3H),6.45-6.45(d,2H,J=1.6),6.51(s,2H),6.83-6.85(d,1H,J=8.4),7.00-7.00(d, 1H, J=1.6), 7.10-7.12(q, 1H).
[0051] HR-ESI Qq-L...
Embodiment 3
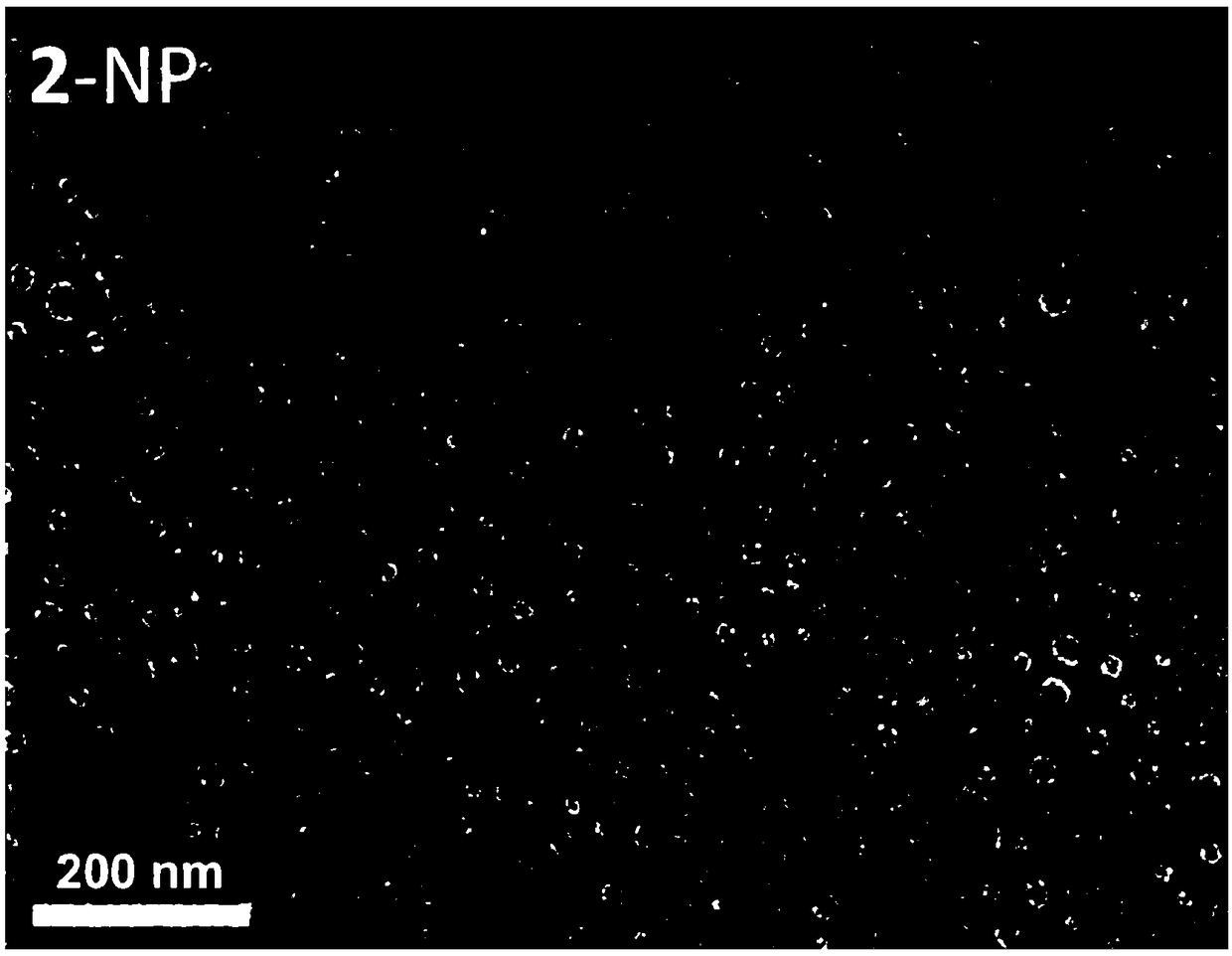
[0053] CA4 (CA4 content 0.5mg) and mPEG 5k -PLA 16k (20 times the mass of the drug) was dissolved in 1 mL of acetone, and evenly added dropwise to 10 mL of water. After the dropwise addition, the acetone was removed under reduced pressure to obtain the CA4 nanomedicine (referred to as 1-NP).
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap