Metal iridium complex, single crystal, benzindole compound, synthesis method and application
A complex, metal iridium technology, applied in the direction of organic compound/hydride/coordination complex catalysts, chemical instruments and methods, compounds containing elements of group 8/9/10/18 of the periodic table, etc., can solve the problem. No literature reports etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
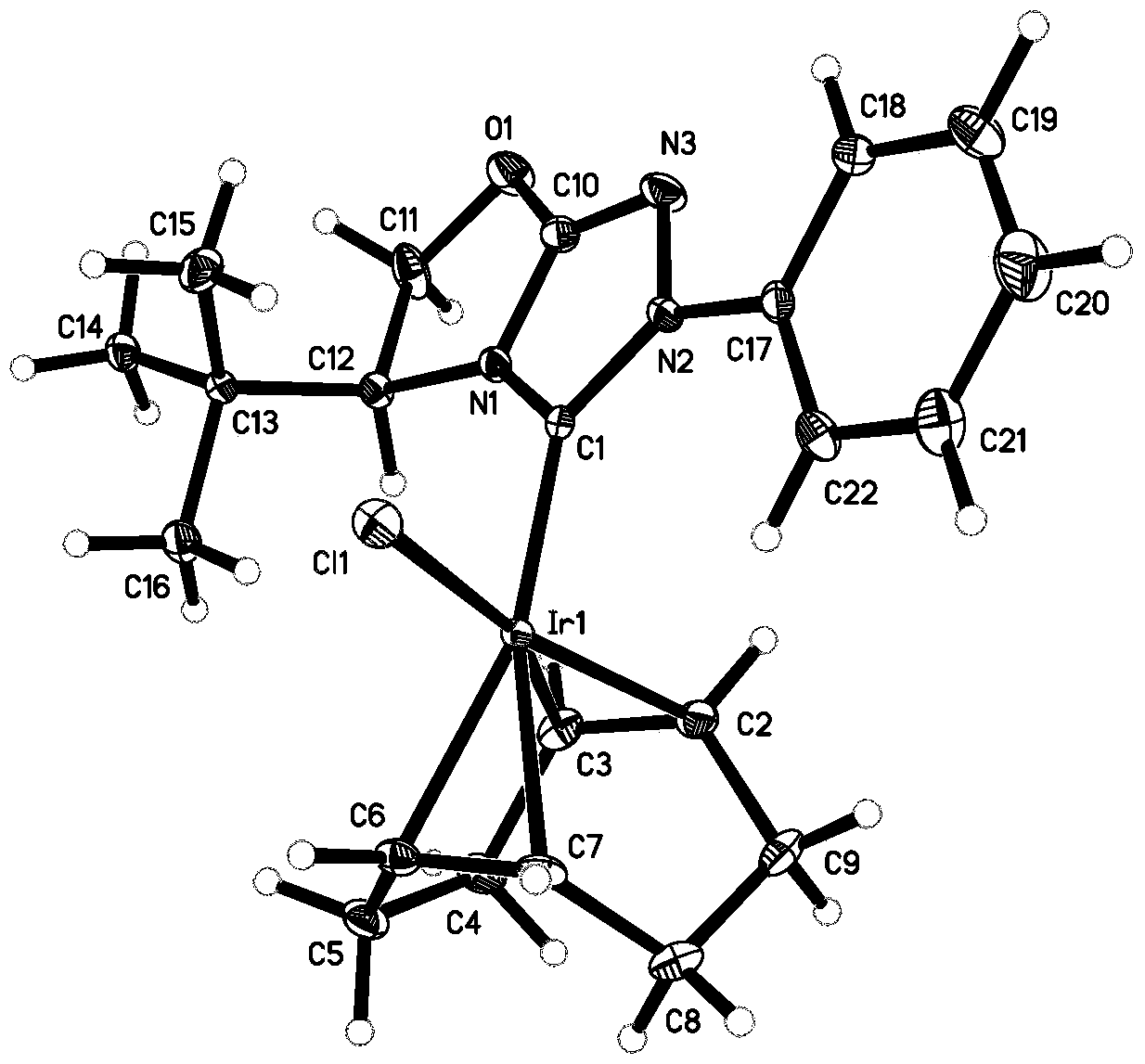
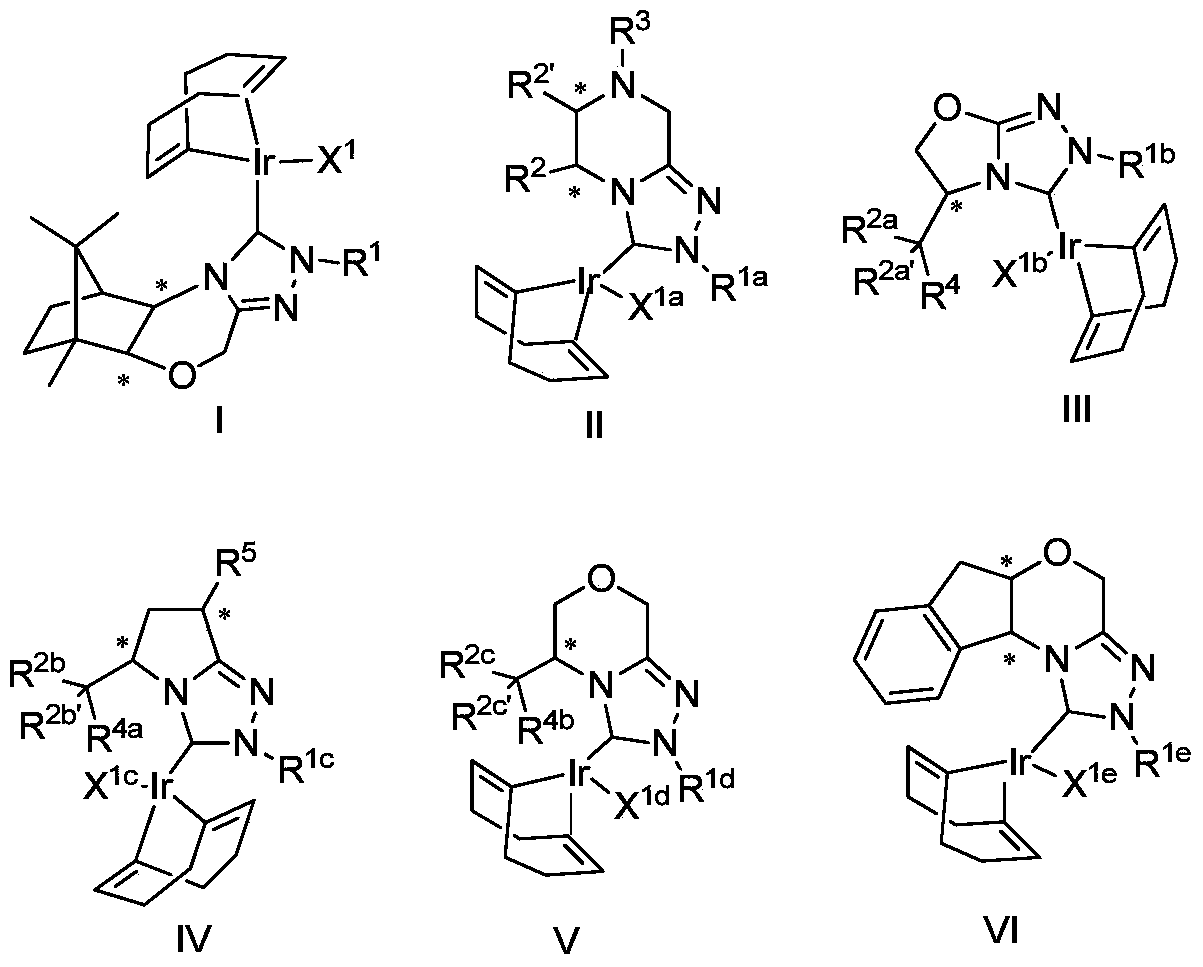
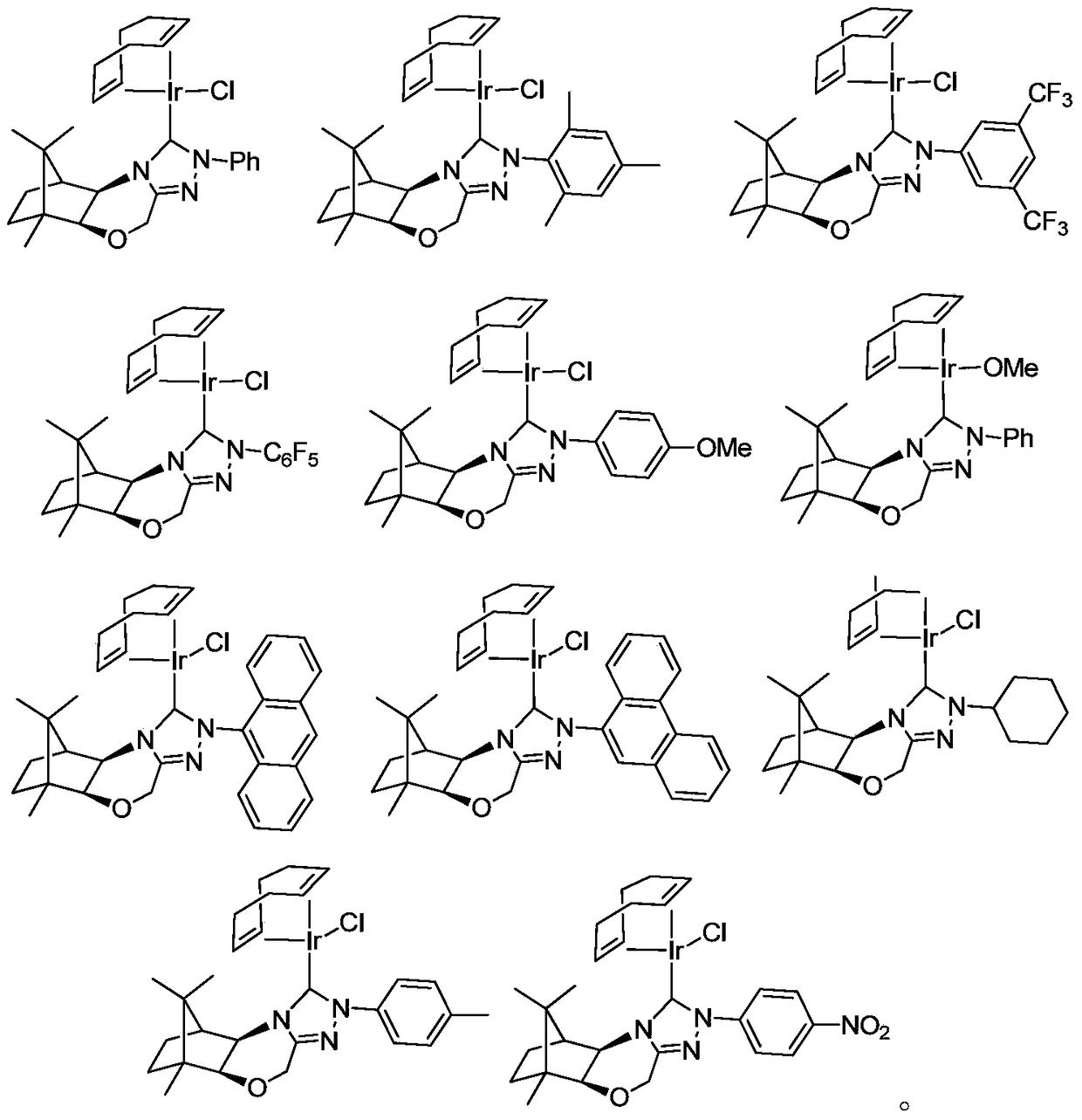
[0085] The synthesis of the metal iridium complex shown in embodiment 1 general formula I
[0086]
[0087] Under argon protection, in a dry 250 ml three-neck flask, add [Ir(cod)Cl] 2 (336 mg, 0.5 mmol), the compound represented by formula a and a base were added with an organic solvent and stirred for 24 hours. The crude product was separated by column chromatography (ethyl acetate / petroleum ether=1:5). See Table 1 for the specific reaction conditions of Compounds Ia-Ic and Compound If. Compound Id-Ie, the preparation method and condition of compound Ig-Ik are with reference to embodiment 1 and table 1, and wherein, the molar ratio in table 1 refers to [Ir(cod) × 1 ] 2 : molar ratio of compound a: base.
[0088] Table 1
[0089]
[0090] Compound Ia
[0091]
[0092] Yellow solid, yield: 54%. (purity>97%, NMR purity)
[0093] 1 H NMR (300MHz, CDCl 3 )δ8.32 (d, J = 7.2Hz, 2H), 7.49-7.46 (m, 3H), 4.94 (AB, J AB =15.0Hz, 1H), 4.80-4.71(m, 3H), 4.44-4.38(m, 2H),...
Embodiment 2
[0148] The synthesis of the metal iridium complex shown in embodiment 2 general formula II
[0149]
[0150] Under argon protection, in a dry 250 ml three-necked bottle, add [Ir(cod)X 1a ] 2 (336mg, 0.5mmol), the compound represented by formula b and a base, an organic solvent, stirred and reacted, and the crude product was separated by column chromatography (ethyl acetate / petroleum ether: 1:5). See Table 2 for the specific reaction conditions of compounds IIa-IIc and compound IIf. Compound IId-IIe, the preparation method and condition of compound IIg-IIu are with reference to embodiment 2 and table 2, wherein, the molar ratio in table 2 refers to [Ir(cod)× 1a ] 2 : molar ratio of compound b: base.
[0151] Table 2
[0152]
[0153] Compound IIa
[0154]
[0155] Yellow solid, yield: 55%. (purity>97%, NMR purity)
[0156] MS (ESI + ):858(C 39 h 42 ikB 4 o 2 S[M + ]).
[0157] Compound IIb
[0158]
[0159] Yellow solid, yield: 56%. (purity>97%, NMR p...
Embodiment 3
[0233] Embodiment 3 is as the synthesis of the metal iridium complex shown in formula III
[0234]
[0235] Under argon protection, in a dry 250 ml three-necked bottle, add [Ir(cod)X 1b ] 2 (336mg, 0.5mmol), the compound represented by formula c and a base, an organic solvent, reacted with stirring. The crude product was separated by column chromatography (ethyl acetate / petroleum ether: 1:5). See Table 3 for the specific reaction conditions of compounds IIIa-IIIc and compound IIIf. The preparation method and condition of compound IIId-IIIe refer to embodiment 3 and table 3, wherein, the molar ratio in table 3 refers to [Ir(cod)× 1b ] 2 : molar ratio of compound c: base.
[0236] table 3
[0237]
[0238]
[0239] MS (ESI + ):1244(C 44 h 45 CIF 17 IrN 4 o 2 S[M + ]).
[0240] Compound IIIa
[0241]
[0242] Yellow solid, yield: 61%. (purity>97%, NMR purity)
[0243] MS (ESI + ):579(C 22 h 29 ikB 3 O[M + ]).
[0244] The preparation method of comp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com