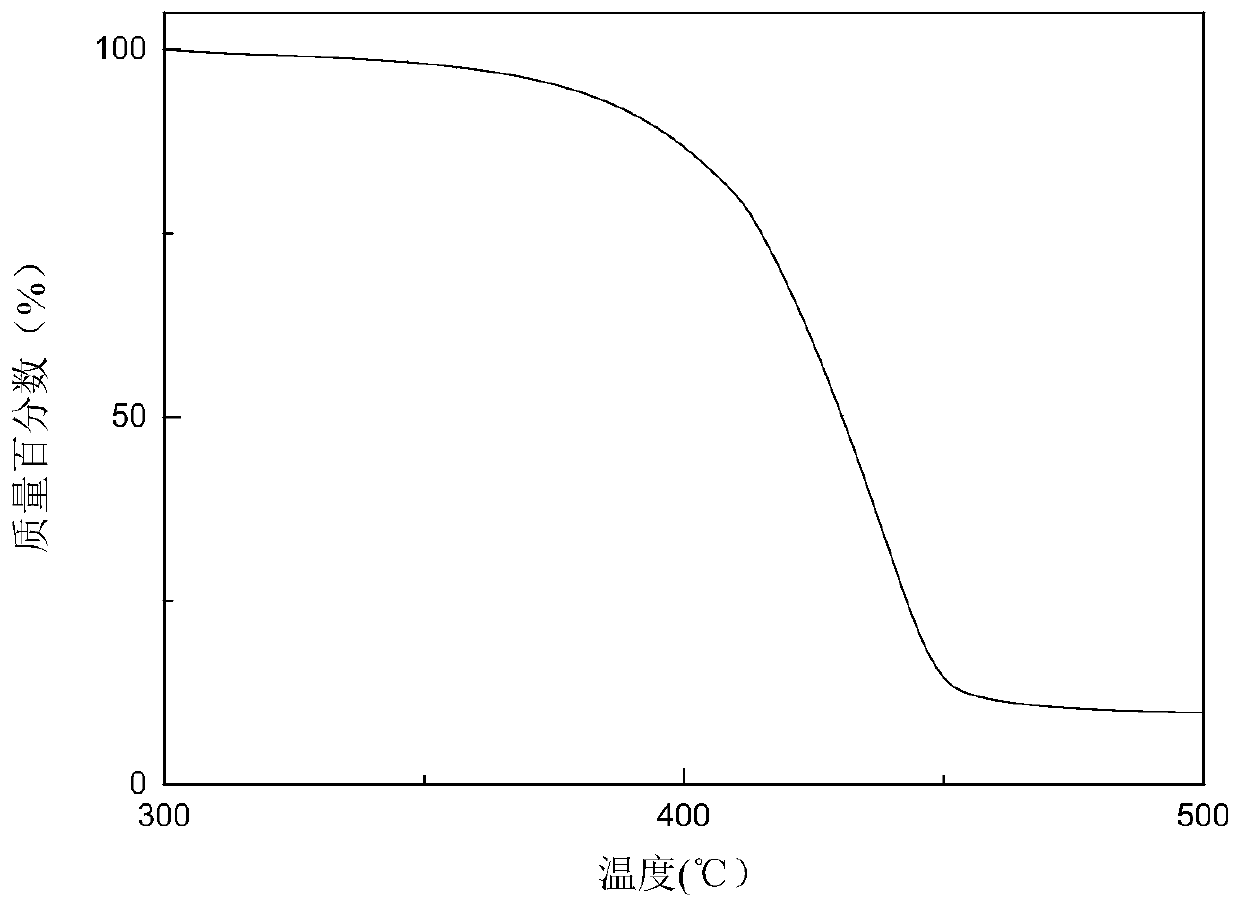
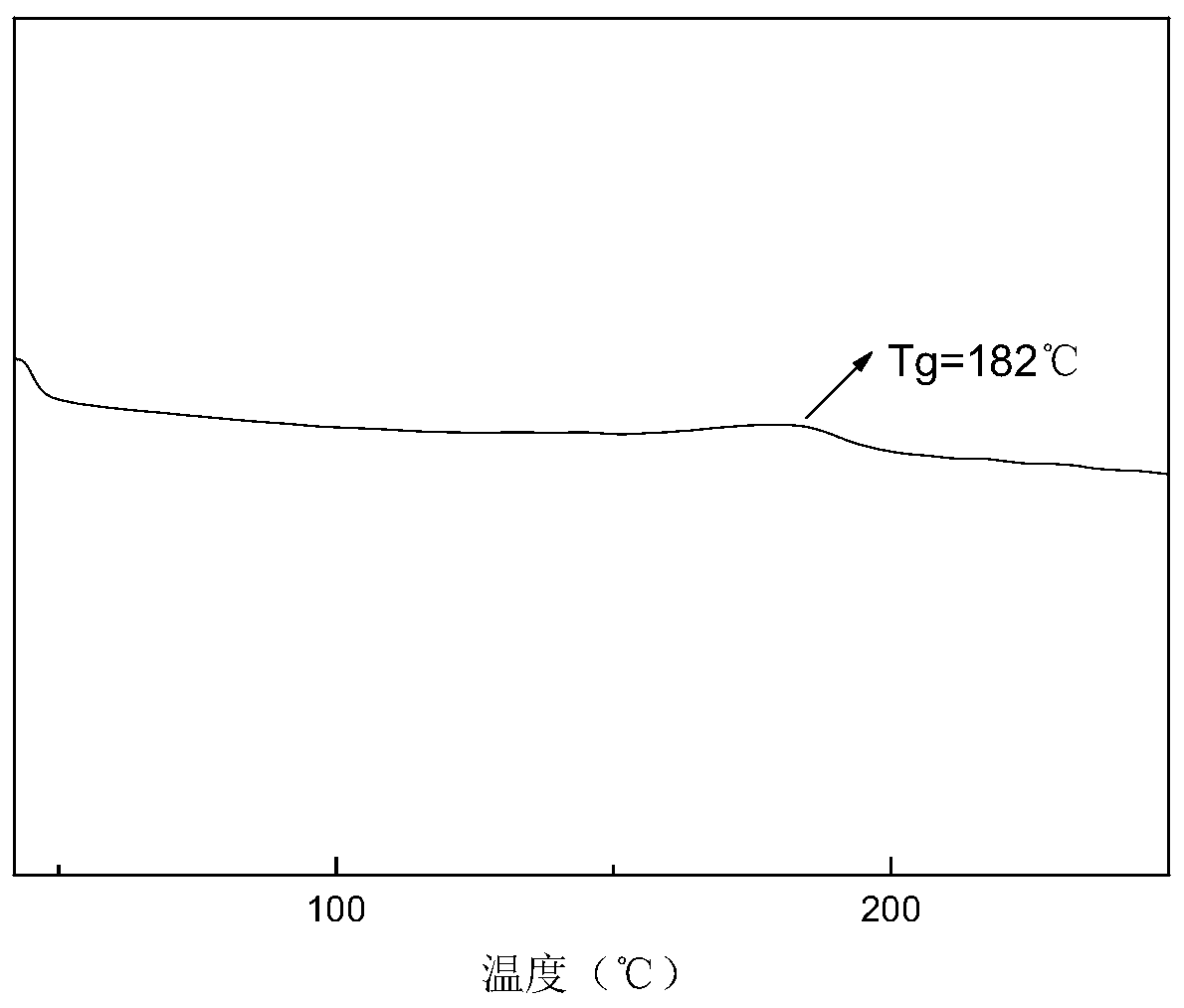
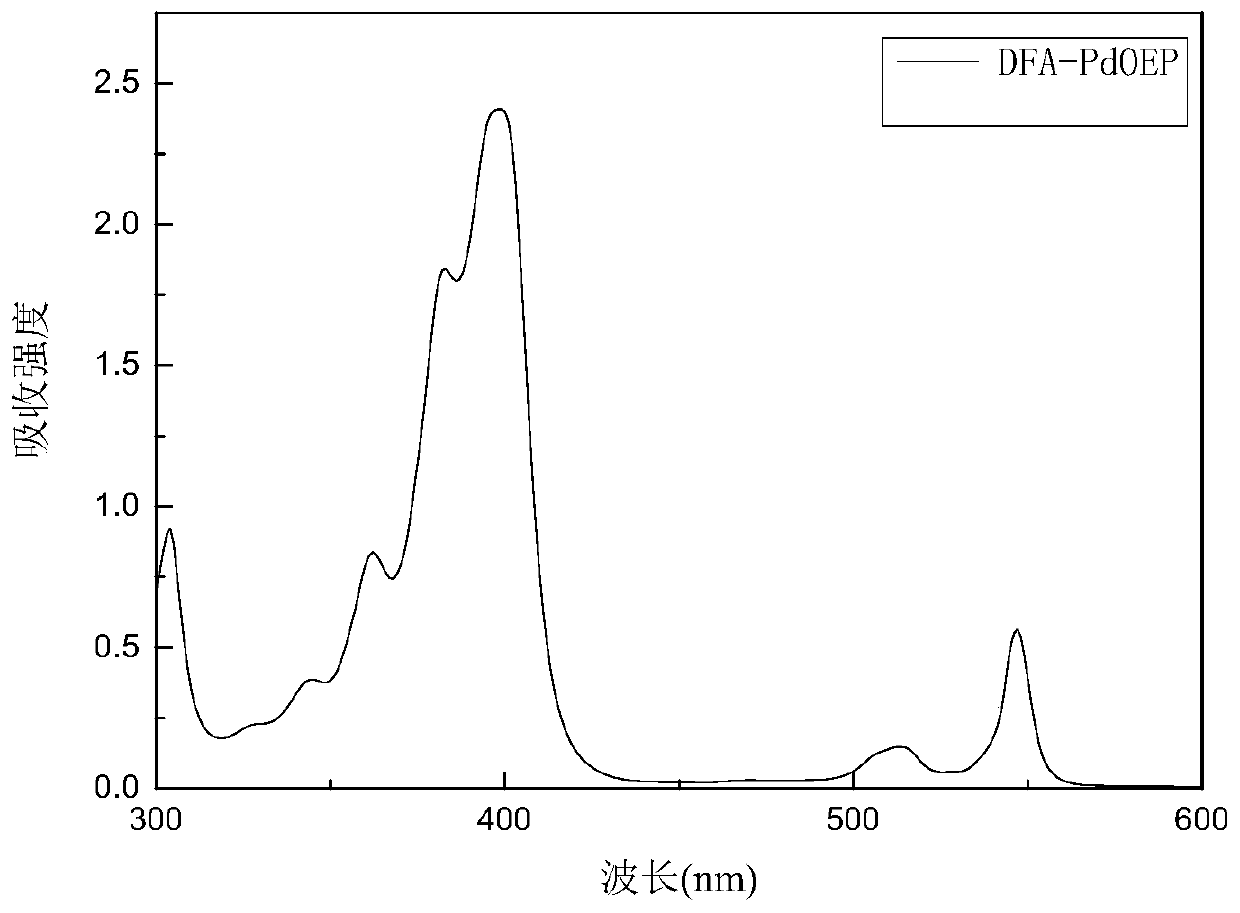
Annihilation agent applied to triplet-triplet annihilation upconversion system and its preparation and application method
A technology of triplet annihilation and triplet state, applied in chemical instruments and methods, condensation between hydrocarbons and non-hydrocarbons, organic chemistry, etc., can solve the problems of low fluorescence quantum yield, few types of annihilation agents, and difficult preparations, etc. problems, to achieve the effect of efficient and stable preparation, no pollution in the preparation process, and easy-to-obtain preparation raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Synthesis of 9,10-Difluorenylanthracene (DFA).
[0031] Respectively, 5mmol of phenylboronic acid, 5mmol of bromobenzaldehyde and 5mmol of K 2 CO 3 Add to 12ml of DMF / H2O solution, stir for 2min and add 5mol% Pd(OAc)2 after fully dissolved, fill with nitrogen protection, stir at 25°C, stop the reaction for 5h, add 50ml of acetic acid to the solution, and wash the ethyl acetate with water The ester was extracted three times, dried with anhydrous magnesium sulfate, and rotary evaporated under reduced pressure to obtain a crude product, which was purified by column chromatography (silica gel, petroleum ether: ethyl acetate 50:1) to obtain a colorless liquid product.
[0032] Add 10mmol 2-formyl biphenyl and 5mmol anthracene into a 250ml single-necked flask equipped with 100ml dichloroethane, stir while adding 10mmol acetic anhydride, drop 0.5mmol trifluoromethanesulfonic acid, stir at 25°C, TLC (developed Petroleum ether:ethyl acetate=30:1) detect the end of the reaction...
Embodiment 2
[0041] Synthesis of 9,10-Difluorenylanthracene (DFA).
[0042] Respectively, 5mmol of phenylboronic acid, 5mmol of bromobenzaldehyde and 5mmol of K 2 CO 3 Add to 12ml of DMF / H2O solution, stir for 2min and add 5mol% Pd(OAc)2 after fully dissolved, fill with nitrogen protection, stir at 25°C, stop the reaction for 8h, add 50ml of acetic acid to the solution, and wash the ethyl acetate with water The ester was extracted three times, dried with anhydrous magnesium sulfate, and rotary evaporated under reduced pressure to obtain a crude product, which was purified by column chromatography (silica gel, petroleum ether: ethyl acetate 50:1) to obtain a colorless liquid product.
[0043] Add 10mmol 2-formyl biphenyl and 5mmol anthracene into a 250ml single-necked flask equipped with 100ml dichloroethane, stir while adding 10mmol acetic anhydride, drop 0.5mmol trifluoromethanesulfonic acid, stir at 25°C, TLC (developed Petroleum ether:ethyl acetate=30:1) detect the end of the reaction...
Embodiment 3
[0045] Synthesis of 9,10-Difluorenylanthracene (DFA).
[0046] Respectively, 5mmol of phenylboronic acid, 5mmol of bromobenzaldehyde and 5mmol of K 2 CO 3 Add it into 12ml DMF / H2O solution, stir for 2min and add 5mol% Pd(OAc)2 after it is fully dissolved, fill it with nitrogen protection, stir at 25°C, stop the reaction for 12h, add 50ml acetic acid to the solution, and wash the acetic acid with water The ester was extracted three times, dried with anhydrous magnesium sulfate, and rotary evaporated under reduced pressure to obtain a crude product, which was purified by column chromatography (silica gel, petroleum ether: ethyl acetate 50:1) to obtain a colorless liquid product.
[0047] Add 10mmol 2-formyl biphenyl and 5mmol anthracene into a 250ml single-necked flask equipped with 100ml dichloroethane, stir while adding 10mmol acetic anhydride, drop 0.5mmol trifluoromethanesulfonic acid, stir at 25°C, TLC (developed Petroleum ether:ethyl acetate=30:1) detect the end of the rea...
PUM
| Property | Measurement | Unit |
|---|---|---|
| conversion efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com