A kind of preparation method of edaravone impurity standard product
A technology of edaravone and standard products, which is applied in the field of medicine, can solve the problems of unreported impurity synthesis and content calibration methods, and achieve the effects of low synthesis cost, short synthesis cycle, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
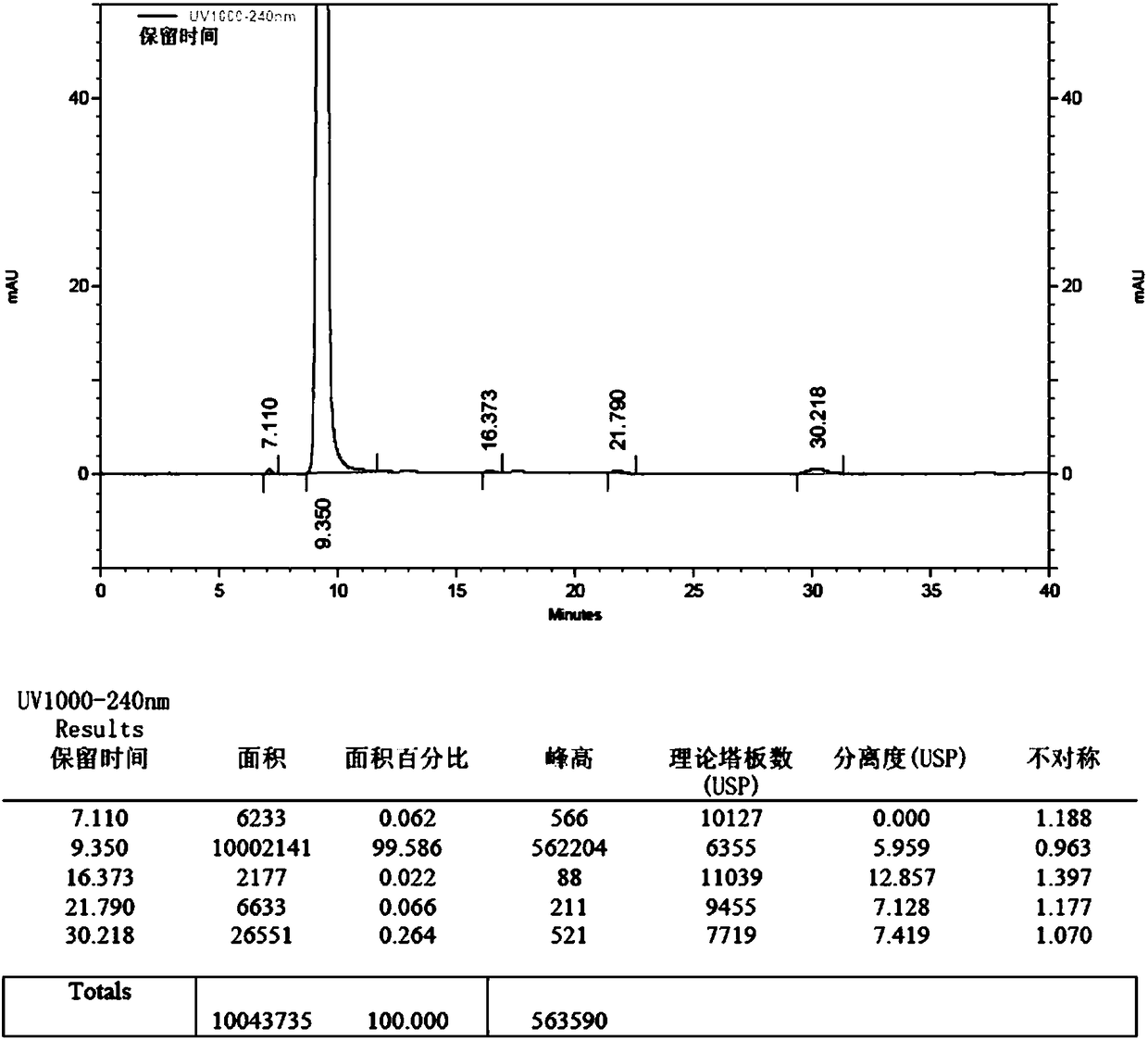
[0043] 1. Put 150ml of chloroform and 10g (57.4mmol) of edaravone into the reaction bottle in sequence. After stirring and dissolving, put in 5g (57.5mmol) of manganese dioxide. Stir and reflux at 65°C for 4 hours; after the reaction, the reaction solution was filtered while hot, and the filtrate was concentrated to dryness at 30-40°C under reduced pressure (vacuum degree ≥ 0.08MPa), to obtain 8.9g of crude impurity product, with a yield of 89.7%.
[0044] 2. Add 100ml of absolute ethanol to 8.9g of crude impurities, stir and reflux at 75-80°C to dissolve, add 0.2g of activated carbon, continue to reflux for 10min, filter while hot, cool the filtrate to 0-10°C, stir and crystallize for 3h, filter , the filter cake was washed with absolute ethanol, and the solid was dried at 40-50°C under reduced pressure (vacuum degree ≥ 0.08MPa) for 6h to obtain 4,4-bis(5-hydroxy-3-methyl-1-phenyl-1H- The pure product of pyrazol-4-yl)-3-methyl-1-phenyl-1H-pyrazol-5(4H)-one was 7.5g, and the y...
Embodiment 2
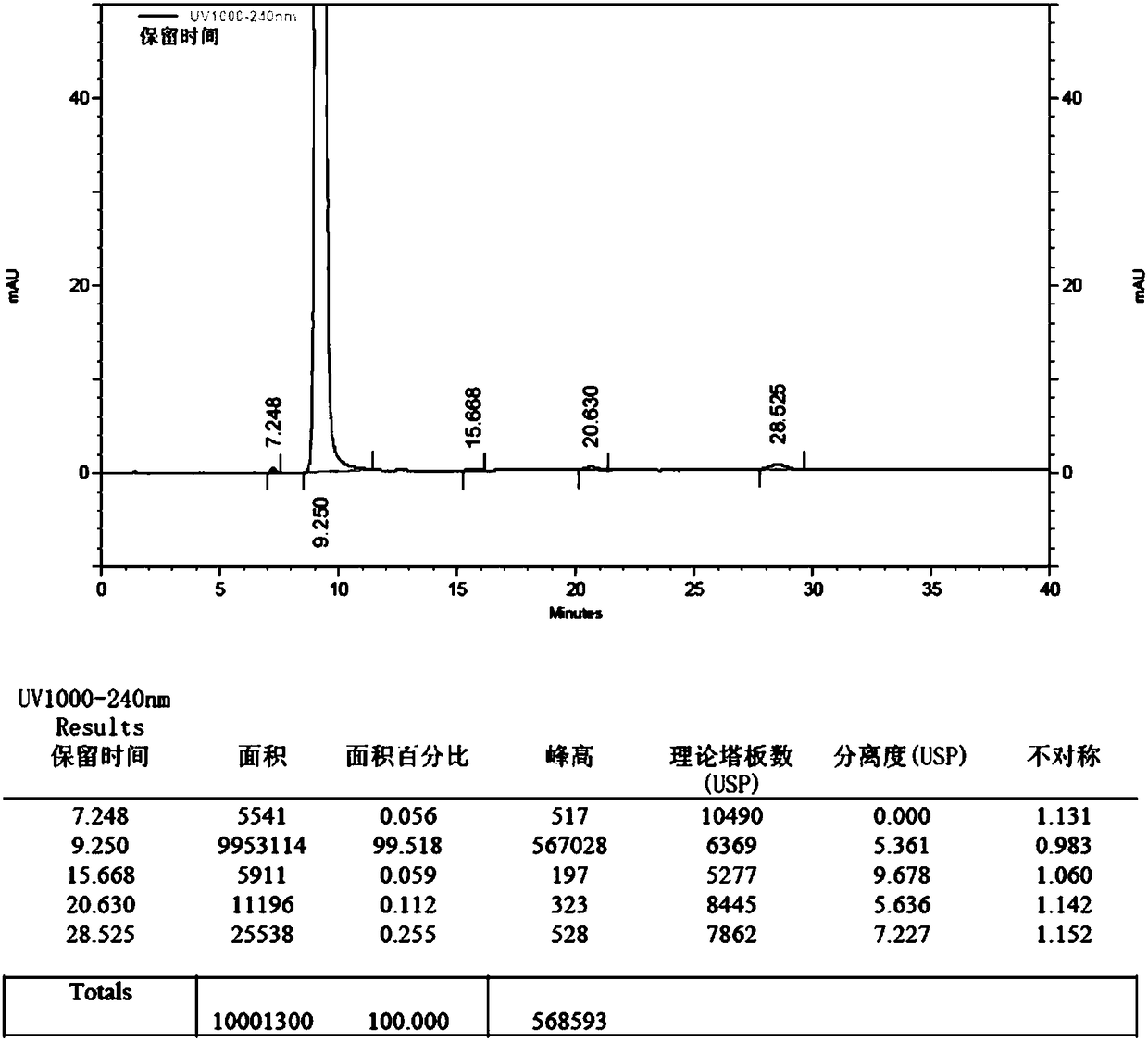
[0054] 1. Put 300ml of chloroform and 20g (114.8mmol) of edaravone into the reaction bottle in sequence. After stirring and dissolving, put in 15g (172.5mmol) of manganese dioxide. Stir and reflux at 65°C for 6h; after the reaction, the reaction solution was filtered while hot, and the filtrate was concentrated to dryness at 30-40°C under reduced pressure (vacuum degree ≥ 0.08MPa) to obtain 18.5g of crude impurity product with a yield of 93.2%.
[0055] 2. Add 350ml of absolute ethanol to 18.5g of impurity crude product, stir and reflux at 75-80°C to dissolve, add 0.5g of activated carbon, continue to reflux for 10min, filter while hot, cool the filtrate to 0-10°C, stir and crystallize for 4h, filter , the filter cake was washed with an appropriate amount of absolute ethanol, and the solid was dried at 40-50°C under reduced pressure (vacuum degree ≥ 0.08MPa) for 8 hours to obtain 4,4-bis(5-hydroxy-3-methyl-1-phenyl-1H -Pyrazol-4-yl)-3-methyl-1-phenyl-1H-pyrazol-5(4H)-one pure ...
Embodiment 3
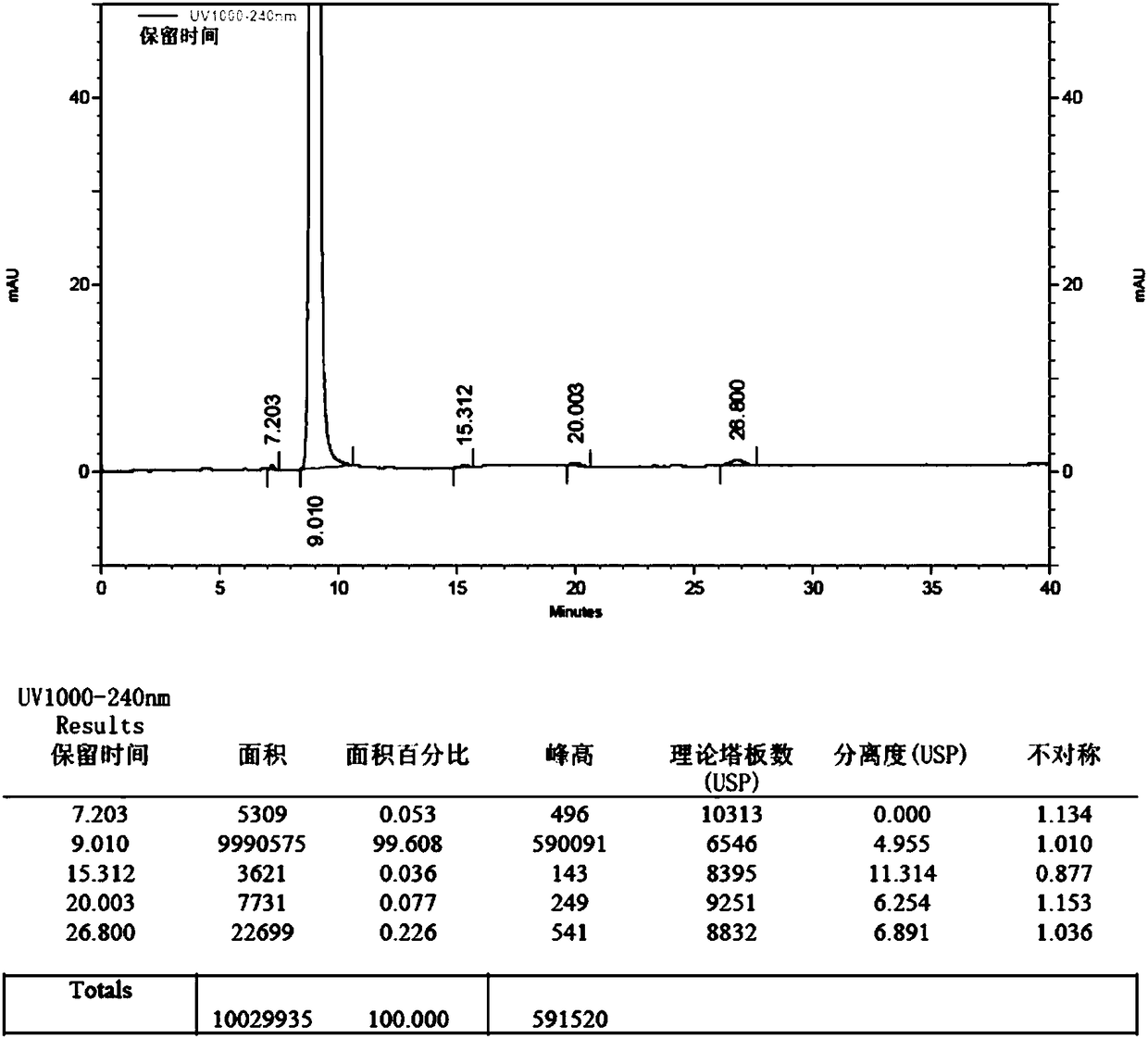
[0059] 1. Put 400ml of chloroform and 50g (287mmol) of edaravone into the reaction bottle in sequence. After stirring and dissolving, put in 75g (863mmol) of manganese dioxide. Stir and reflux for 8 hours; after the reaction, the reaction solution was filtered while it was hot, and the filtrate was concentrated to dryness at 30-40°C under reduced pressure (vacuum degree ≥ 0.08MPa) to obtain 47.3g of crude impurities.
[0060] Yield 95.3%.
[0061] 2. Add 700ml of absolute ethanol to 47.3g of impurity crude product, stir and reflux at 75-80°C to dissolve, add 4.5g of activated carbon, continue to reflux for 10min, filter while hot, cool the filtrate to 0-10°C, stir and crystallize for 4h, filter , the filter cake was washed with an appropriate amount of absolute ethanol, and the solid was dried at 40-50°C under reduced pressure (vacuum degree ≥ 0.08MPa) for 8 hours to obtain 4,4-bis(5-hydroxy-3-methyl-1-phenyl-1H -Pyrazol-4-yl)-3-methyl-1-phenyl-1H-pyrazol-5(4H)-one pure produ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com