A kind of synthetic method of macitentan impurity standard substance
A synthesis method and technology of macitentan, applied in the field of medicine, can solve the problems such as unreported synthesis and content calibration methods, and achieve the effects of low synthesis cost, short synthesis period and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
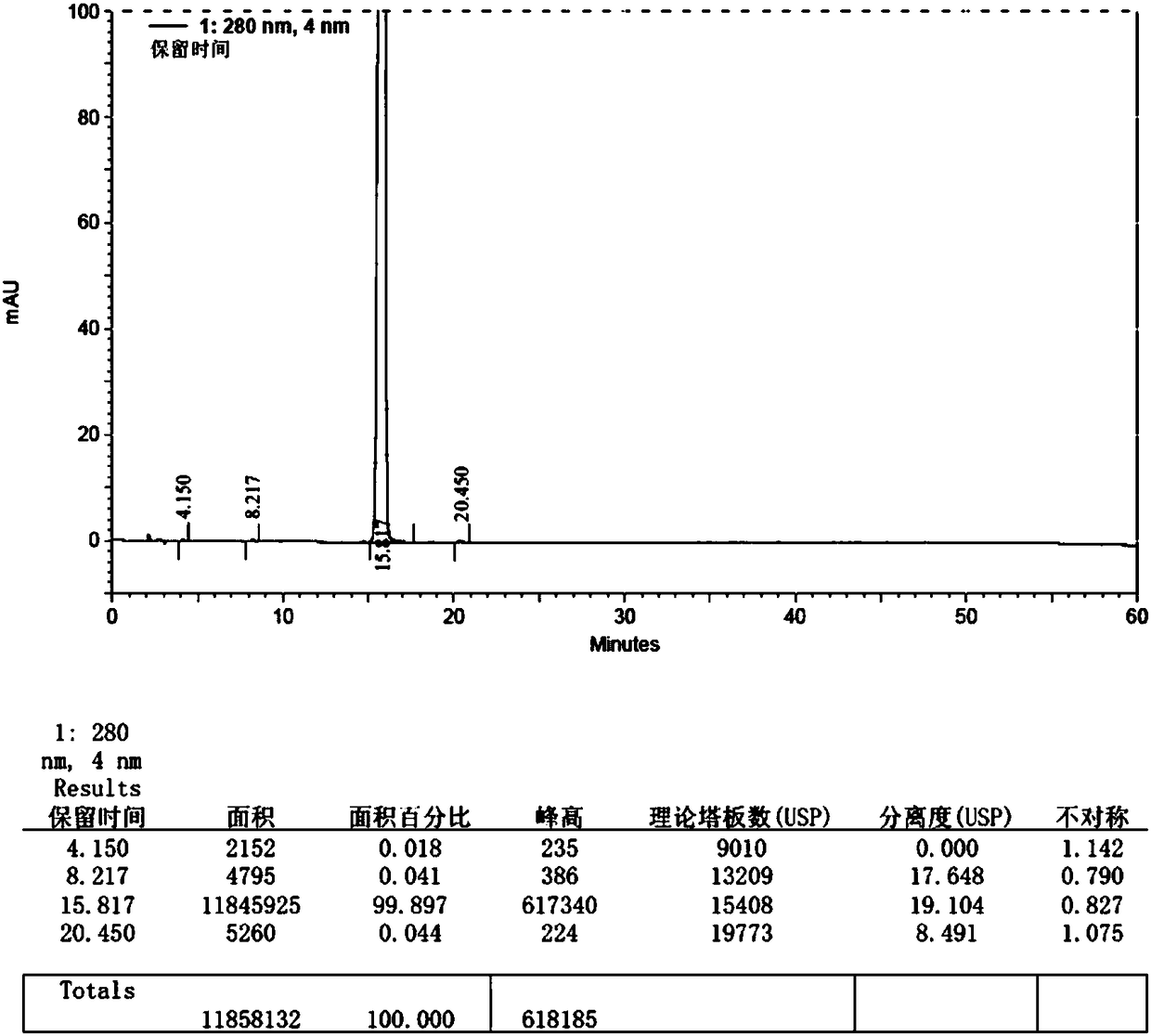
Embodiment 1
[0046] The synthetic method of macitentan impurity standard substance in the present embodiment is as follows:
[0047] 1. Add 20g (34mmol) of macitentan into 120ml ethanol, heat and stir to dissolve, add 50ml (125mmol) of 10% sodium hydroxide aqueous solution dropwise, heat and stir at 70-80°C for 6h, cool down to 0- 10°C, pour the reaction solution into 480ml (125mmol) of 5% citric acid aqueous solution, control the temperature at 0-10°C, stir for 1h, filter, wash the filter cake with purified water until the filtrate is neutral, collect the solid, and put it at 50-55°C After drying under reduced pressure (vacuum degree: 0.096 MPa) at ℃ for 6 hours, 12.2 g of impurity crude product was obtained, yield: 76.82%.
[0048]2. Add 120ml of ethanol to 12.2g of impurity crude product, reflux at 75-85°C and stir to dissolve, filter while hot, slowly cool the filtrate to 0-5°C, stir and crystallize for 4 hours, filter, and use an appropriate amount of ethanol at 0-5°C for the filter c...
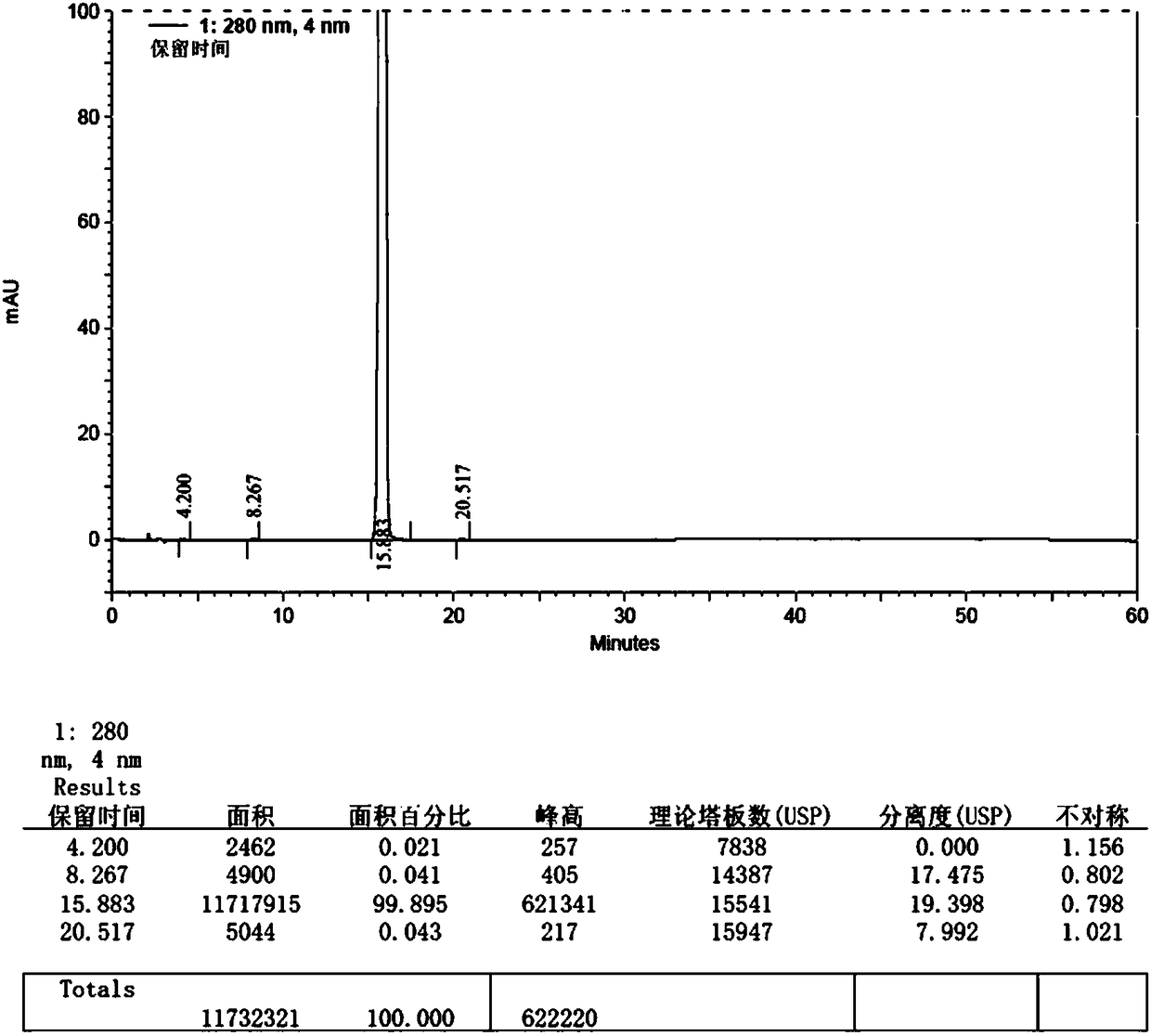
Embodiment 2
[0058] The synthetic method of macitentan impurity standard substance in the present embodiment is as follows:
[0059] 1. Add 20g (34mmol) of macitentan into 150ml ethanol, heat and stir to dissolve, add dropwise 70ml (125mmol) of 10% potassium hydroxide aqueous solution, heat and stir at 70-80°C for 7h, and cool down to 0- 10°C, pour the reaction solution into 500ml (130mmol) of 5% citric acid aqueous solution, control the temperature at 0-10°C, stir for 1h, filter, wash the filter cake with purified water until the filtrate is neutral, collect the solid, and put it at 50-55°C After drying under reduced pressure (vacuum degree: 0.097 MPa) at ℃ for 8 hours, 11.8 g of impurity crude product was obtained, yield: 74.30%.
[0060] 2. Add 120ml of ethanol to 11.8g of impurity crude product, reflux at 75-85°C and stir to dissolve, filter while hot, slowly cool the filtrate to 0-5°C, stir and crystallize for 3 hours, filter, and use an appropriate amount of ethanol at 0-5°C for the ...
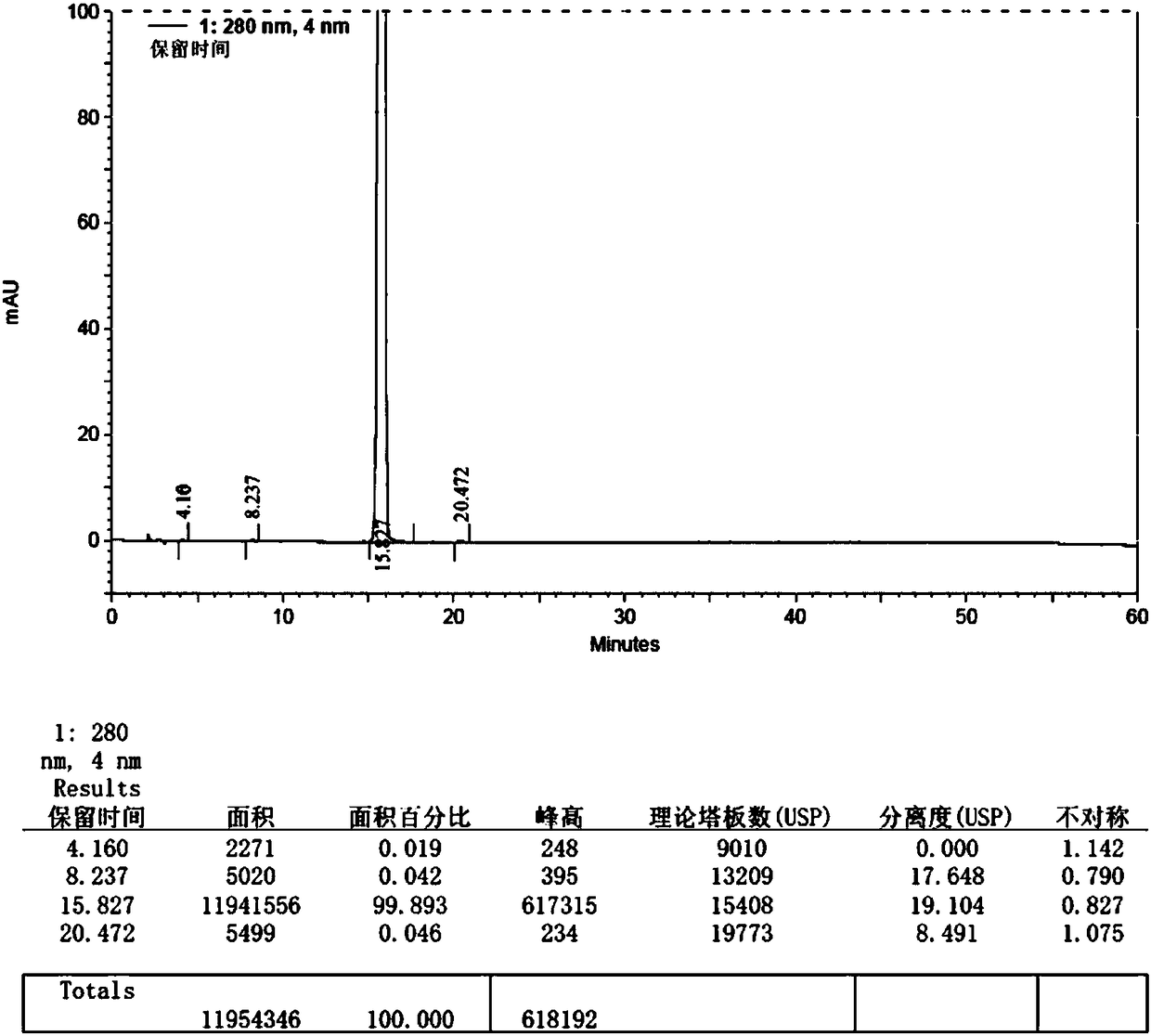
Embodiment 3
[0064] The synthetic method of macitentan impurity standard substance in the present embodiment is as follows:
[0065] 1. Add 20g (34mmol) of macitentan into 200ml ethanol, heat and stir to dissolve, add 68ml (170mmol) of 10% aqueous sodium hydroxide solution dropwise, heat and stir at 70-80°C for 6h, and cool down to 0- 10°C, pour the reaction solution into 780ml (203mmol) of 5% citric acid aqueous solution, control the temperature at 0-10°C, stir for 1h, filter, wash the filter cake with purified water until the filtrate is neutral, collect the solid, and put it at 50-55°C ℃ and dried under reduced pressure (vacuum degree 0.092MPa) for 7 hours to obtain 12.8 g of crude impurity product, yield: 80.60%.
[0066] 2. Add 150ml of ethanol to 12.8g of crude impurity product, reflux at 75-85°C and stir to dissolve, filter while hot, slowly cool the filtrate to 0-5°C, stir and crystallize for 3 hours, filter, and use an appropriate amount of ethanol at 0-5°C for the filter cake Wa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com