Method for synthesizing standard substance of degradation impurity of bromfenac sodium sesquihydrate
A synthesis method, the technology of bromfenac sodium, is applied in the field of medicine to achieve the effects of simple preparation process, low synthesis cost and short synthesis cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1: Preparation of 7-(4-bromobenzoyl)indoline-2,3-dione
[0044] 7-(4-bromobenzoyl)indolin-2-one (CAS No. 91713-91-6) prepared by a general industrial method is used as a raw material.
[0045] Dissolve 25g (79mmol) of 7-(4-bromobenzoyl)indolin-2-one in 750ml of ethyl acetate with stirring, add 88.2g (395mmol) of copper bromide, and react under reflux at 78-85°C After 4 hours, the temperature was lowered to 20-30°C, the reaction solution was washed successively with water equal to the volume of ethyl acetate and saturated aqueous sodium chloride solution, the organic phase was concentrated to dryness at 50-60°C under reduced pressure, and methanol / water (volume ratio 4 : 1) 700ml of mixed solution, reflux at 70-80°C and stir for 3h, cool to 20-30°C, filter, wash the filter cake with appropriate amount of water and methanol successively, add 500ml of methanol to the solid, reflux at 65-70°C for 30min, Filtrate hot, cool the filtrate to -5~0°C, stir and crystalliz...
Embodiment 2
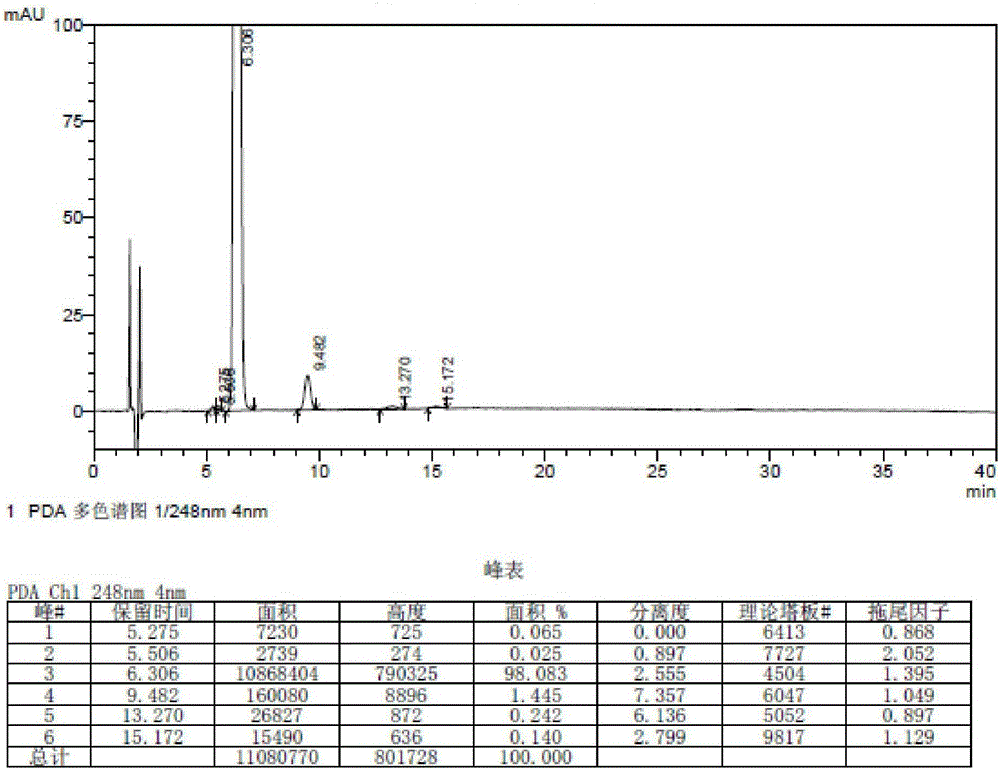
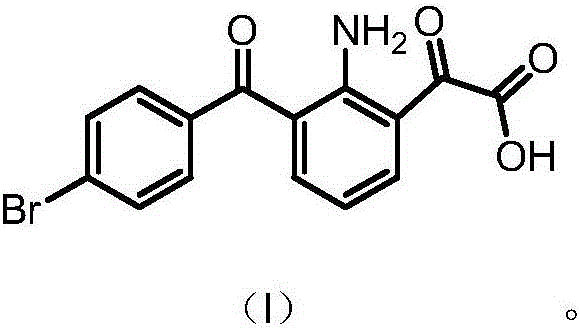
[0052] Example 2: Preparation of 2-(2-amino-3-(4-bromobenzoyl)phenyl)-2-oxoacetic acid
[0053]1. Add 8.25g (25mmol) of 7-(4-bromobenzoyl)indoline-2,3-dione into 75ml (75mmol) of 1N potassium hydroxide solution, stir and heat up to 50-60°C, Stir the reaction for 6h, stir and cool down to 20-30°C, filter, adjust the pH of the filtrate to 3-4 with dilute hydrochloric acid, stir for 0.5h, filter, wash with appropriate amount of water, and dry the solid at 50-60°C under reduced pressure for 4-6h to obtain 2- (2-Amino-3-(4-bromobenzoyl)phenyl)-2-oxoacetic acid crude product 7g, yield 80.5%;
[0054] 2. Add 7g of crude 2-(2-amino-3-(4-bromobenzoyl)phenyl)-2-oxoacetic acid into 100ml of ethanol, stir and heat up to 75-80°C to dissolve, and filter while hot. The filtrate was cooled to 0-10°C, stirred and crystallized for 3-4 hours, filtered, and the solid was dried under reduced pressure at 50-60°C for 4-6 hours to obtain 2-(2-amino-3-(4-bromobenzoyl)phenyl )-2-oxoacetic acid pure p...
Embodiment 3
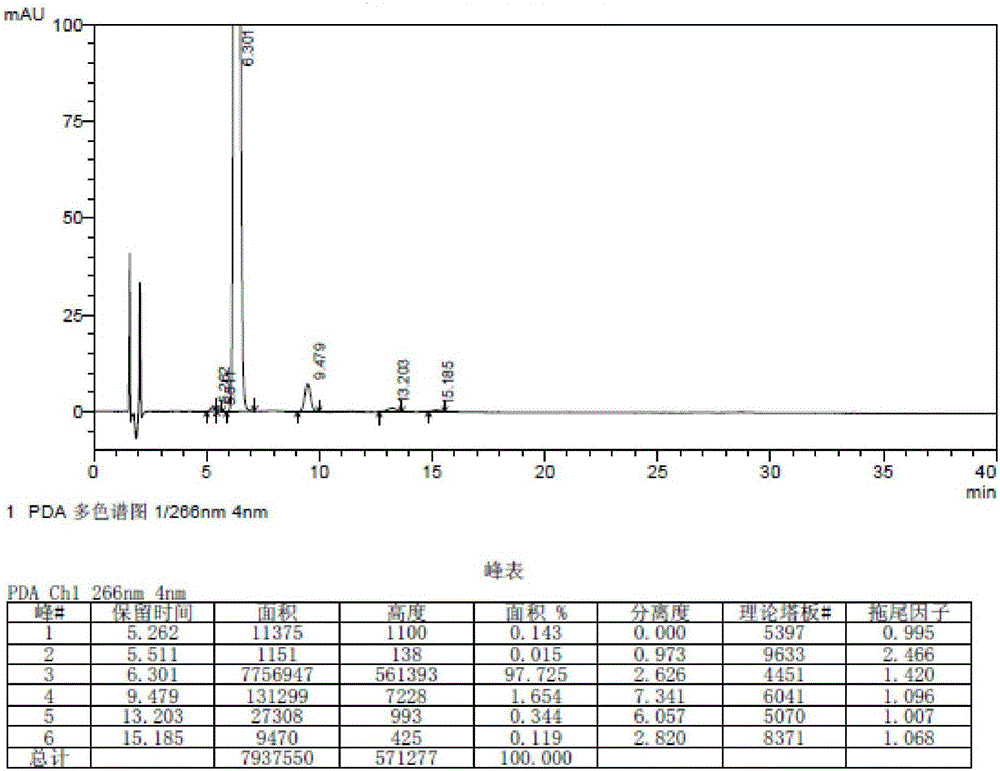
[0064] Example 3: Preparation of 2-(2-amino-3-(4-bromobenzoyl)phenyl)-2-oxoacetic acid
[0065] 1. Add 10g (30.2mmol) of 7-(4-bromobenzoyl)indoline-2,3-dione into 120ml (120mmol) of 1N potassium hydroxide solution, stir and heat up to 50-60°C, Stir the reaction for 6h, stir and cool down to 20-30°C, filter, adjust the pH of the filtrate to 3-4 with dilute hydrochloric acid, stir for 0.5h, filter, wash with appropriate amount of water, and dry the solid at 50-60°C under reduced pressure for 4-6h to obtain 2- (2-Amino-3-(4-bromobenzoyl)phenyl)-2-oxoacetic acid crude product 8.3g, yield 78.9%;
[0066] 2. Add 8.3g of crude 2-(2-amino-3-(4-bromobenzoyl)phenyl)-2-oxoacetic acid into 160ml of acetonitrile, stir and heat up to 75-80°C to dissolve, and filter while hot , the filtrate was cooled to 0-10°C, stirred and crystallized for 3-4h, filtered, and the solid was dried under reduced pressure at 50-60°C for 4-6h to obtain 2-(2-amino-3-(4-bromobenzoyl)benzene Base)-2-oxoacetic aci...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com