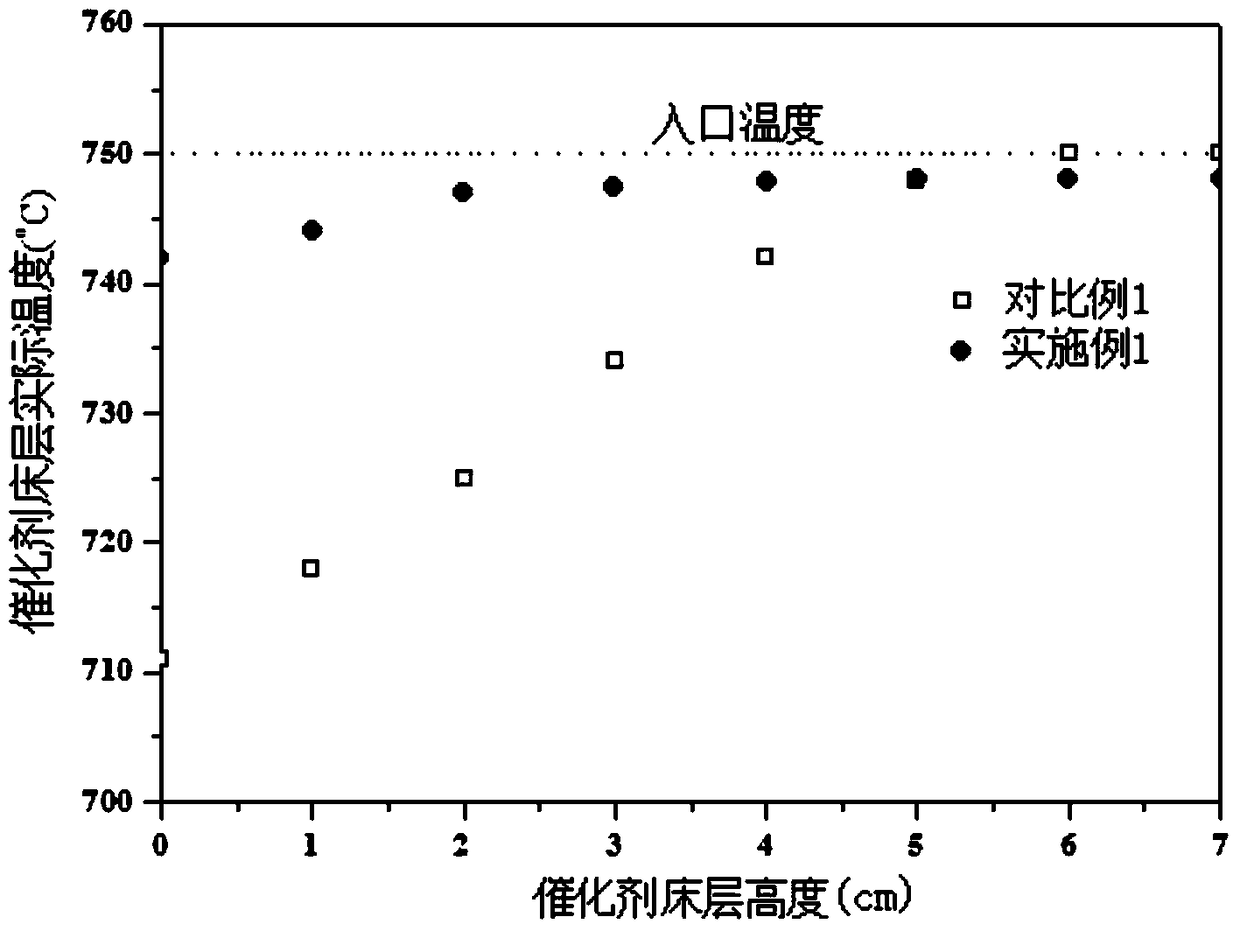
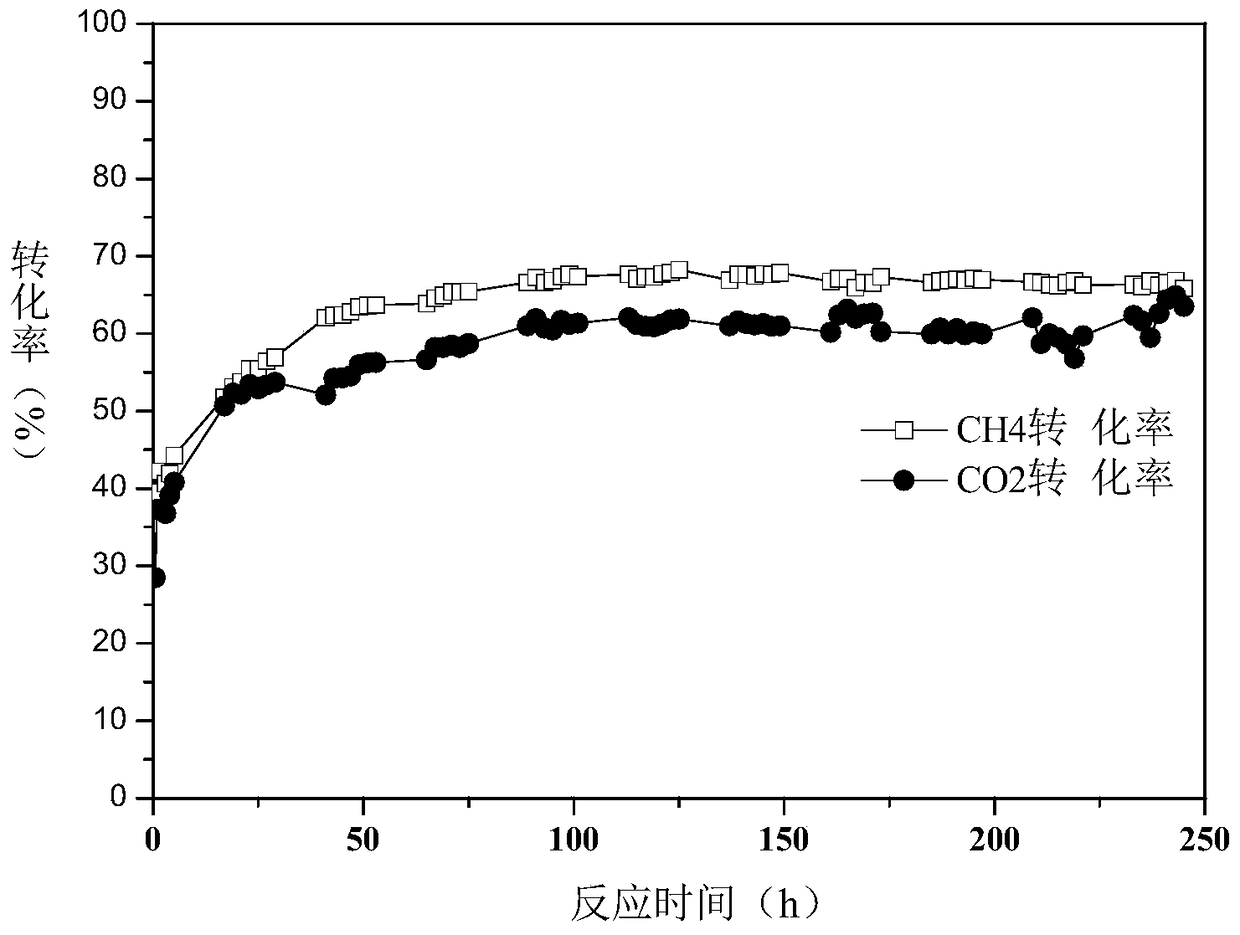
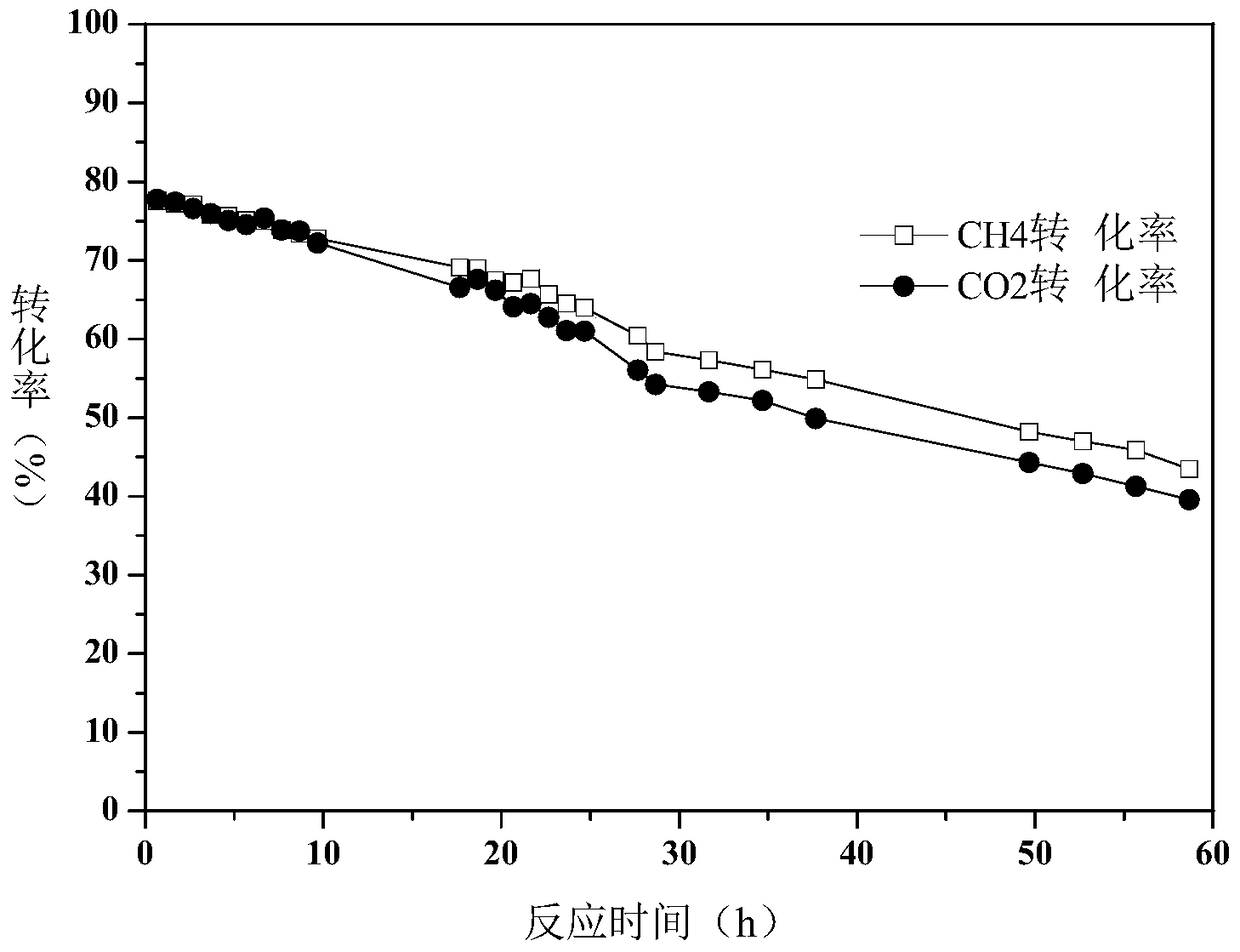
A method for producing synthesis gas by dry reforming of methane
A methane dry reforming and synthesis gas technology, which is applied in chemical instruments and methods, inorganic chemistry, bulk chemical production, etc., can solve the problems of poor anti-coking performance of catalysts, short service life of catalysts, and low reaction space velocity, etc. Achieve the effect of improving catalytic stability, uniform temperature distribution and high catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0023] According to a preferred embodiment of the present invention, the carrier is La and / or Mg modified Al 2 o 3 carrier, and relative to 100 parts by weight of Al modified with additives 2 o 3 As for the carrier, the content of additives La and / or Mg is 3-13 parts by weight, preferably 6-12 parts by weight, based on oxides.
[0024] According to a preferred embodiment of the present invention, relative to 100 parts by weight of the catalyst, the content of the active metal component is 4.5-6.5 parts by weight in terms of metal elements.
[0025] In the present invention, the impregnation method in the carrier modification method is not particularly limited, for example, it can be an equal-volume impregnation method commonly used in the art, or a supersaturated impregnation method, or a combination of the above methods. The temperature and time of the immersion treatment are not particularly limited, as long as the desired auxiliary ingredients can be loaded on the carrie...
Embodiment 1
[0043] This example is used to illustrate the method for producing synthesis gas by dry reforming of methane provided by the present invention.
[0044] (1) Preparation of catalyst carrier
[0045] Weigh 5.53g of Mg(NO 3 ) 2 ·6H 2 O was dissolved in 14g of deionized water to prepare carrier impregnation solution; after the dissolution was completed, it was impregnated in 10g of Al 2 o 3 On the carrier, the immersion temperature is 25°C, and it is allowed to stand for 2 hours. The strips are then extruded on an extruder. The obtained wet strip was dried at 120° C. for 8 hours, and then put into a muffle furnace for sintering at 800° C. for 3 hours. The resulting support is denoted as 8Mg-Al 2 o 3 .
[0046] (2) Preparation of catalyst
[0047] Weigh 1.06g of Ni(NO 3 ) 2 ·6H 2 O and 0.211g of P123 were dissolved in 5.2g of deionized water and stirred to dissolve, and the impregnating solution was impregnated in 4g of the above carrier 8Mg-Al 2 o 3 After standing f...
Embodiment 2
[0051] This example is used to illustrate the method for producing synthesis gas by dry reforming of methane provided by the present invention.
[0052] (1) Preparation of catalyst carrier
[0053] Weigh 1.7g of La(NO 3 ) 3 ·6H 2 O was dissolved in 14g of deionized water to prepare carrier impregnation solution; after the dissolution was completed, it was impregnated in 10g of Al 2 o 3 On the carrier, the immersion temperature is 35°C, and it is allowed to stand still for 1 hour. The strips are then extruded on an extruder. The obtained wet strip was dried at 90° C. for 10 hours, and then put into a muffle furnace for sintering at 1000° C. for 2 hours. The resulting support is denoted as 6La-Al 2 o 3 .
[0054] (2) Preparation of catalyst
[0055] Weigh 0.84g of Ni(NO 3 ) 2 ·6H 2 O and 0.166g of P123 were put into 5.2g of deionized water and stirred and dissolved; this catalyst impregnation solution was impregnated in 4g of the above-mentioned carrier 6La-Al 2 o ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com