Synthetic method of proline cyclic dipeptide
A synthetic method and technology of cyclic dipeptides, which are applied in the field of synthetic cyclic dipeptides, can solve problems such as the influence of product optical purity, and achieve the effect of avoiding changes in optical purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0027] According to the first specific embodiment of the present invention, the method for synthesizing cyclic dipeptides of the present invention comprises the following steps:
[0028] (1) Preparation of amino-protected progroup dipeptide methyl ester
[0029] Preparation of amino-protected pro group dipeptide methyl ester from histidine methyl ester hydrochloride and amino-protected proline. Specifically, proline whose amino group is protected by fluorenyl moxycarbonyl and N-hydroxysuccinimide generate active ester under the action of N,N'-dicyclohexylcarbodiimide, and then further react with histidine methyl ester Hydrochloride under alkaline conditions (for example, NaHCO 3 In the presence of) react to generate the pro group dipeptide methyl ester whose amino group is protected by fluorenylmethoxycarbonyl.
[0030] Among them, the molar ratio of proline whose amino group is protected by fluorenyl moxycarbonyl, N-hydroxysuccinimide, N,N'-dicyclohexylcarbodiimide, and his...
Embodiment 1
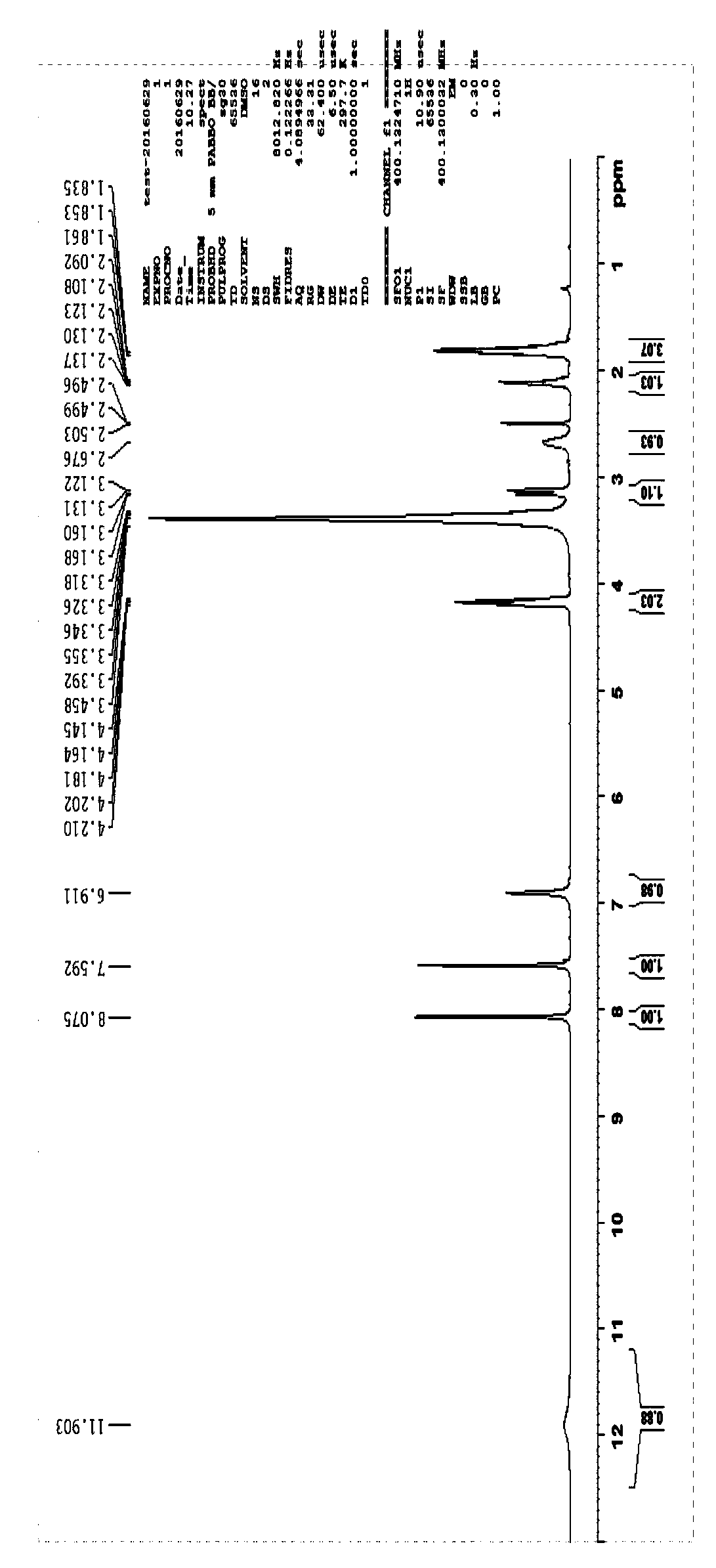
[0055] Example 1 L-L type proline group cyclic dipeptide
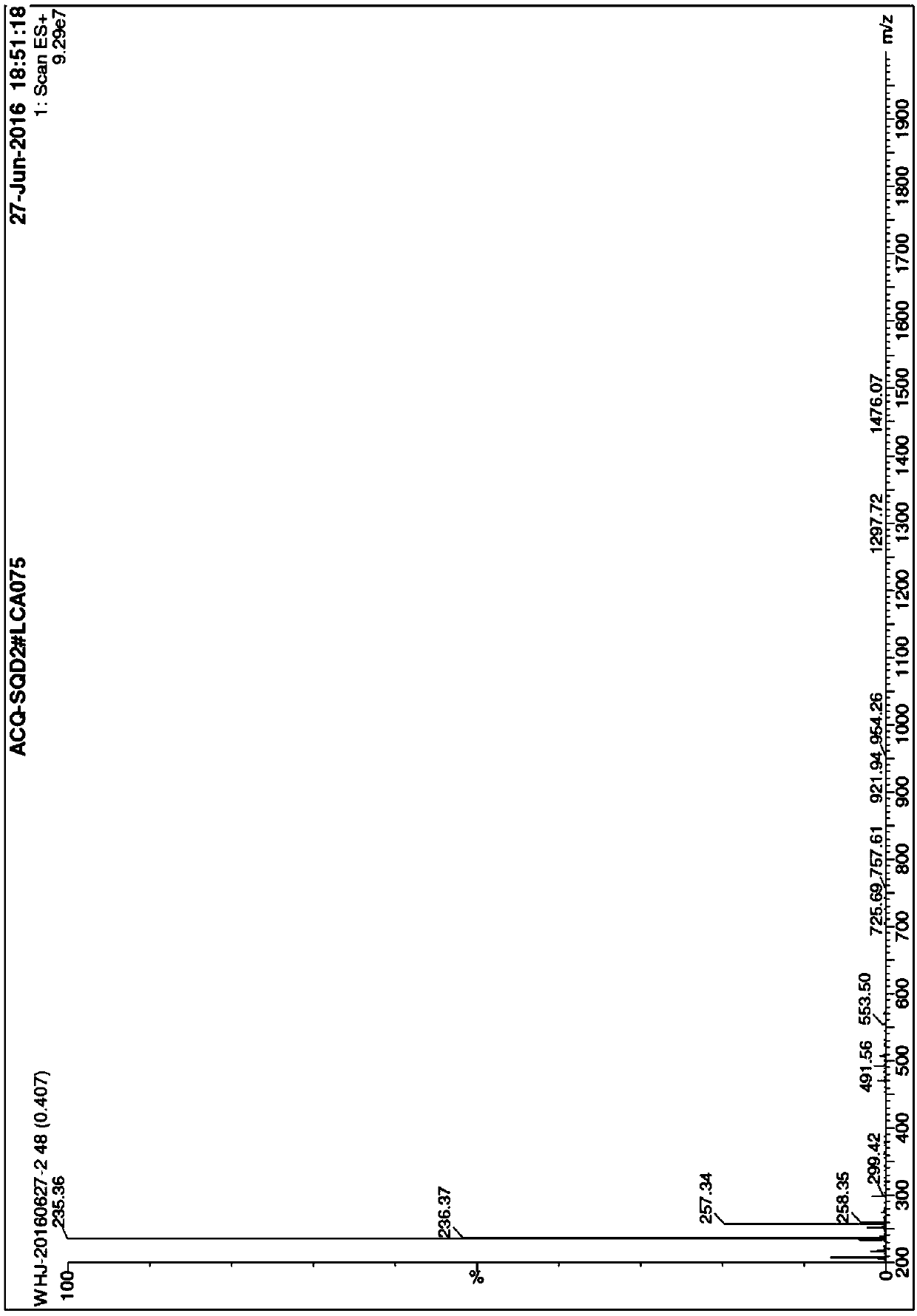
[0056] 1. Add Fmoc-L-Pro-OH (30g, 0.08mol), 500mlTHF into an eggplant-shaped bottle, stir magnetically, and stir to dissolve it all, then add HOSu (10.2g, 0.09mol), DCC (18.3g , 0.09mol), stirred overnight at room temperature, a large amount of white precipitate appeared, filtered, took the filtrate, added THF to make up the volume of 500ml, stirred at room temperature, and added L-H-His-OCH to the filtrate respectively 3 .2HCl (23g, 0.096mol), NaHCO 3 (30g, 0.36mol), H 2 O150ml, stirred overnight at room temperature, TLC showed that the reaction was complete, treated, evaporated THF to dryness, added ethyl acetate and water, stirred, separated layers, took the ethyl ester layer, extracted the water layer with ethyl ester once, poured it off, and combined the ethyl ester layers , the ethyl ester layer was washed twice with dilute hydrochloric acid aqueous solution to remove excess H-His-OCH 3 , washed 3 times with b...
Embodiment 2
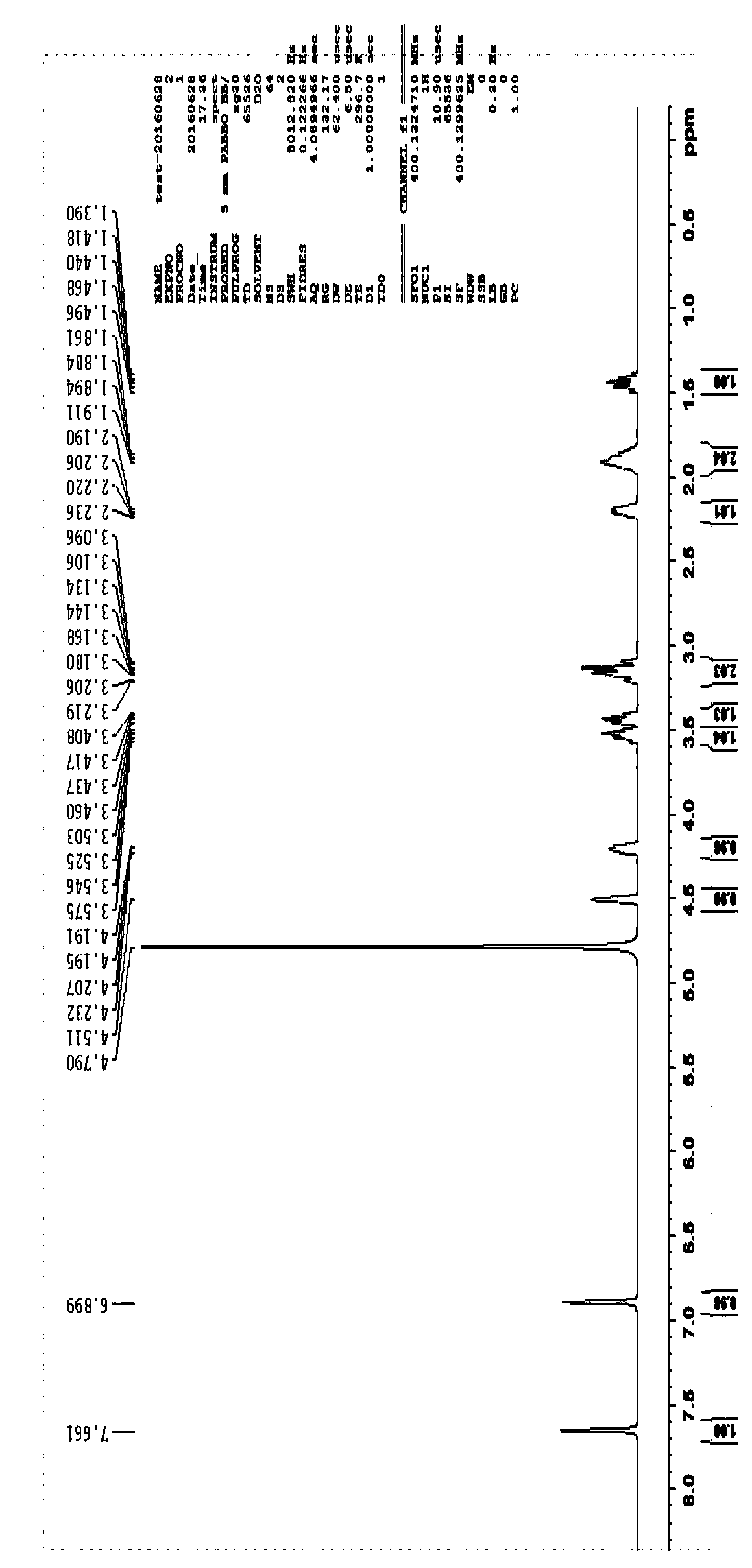
[0058] Example 2 L-D type proline group cyclic dipeptide
[0059] 1. Add Fmoc-L-Pro-OH (30g, 0.08mol), 500mlTHF into an eggplant-shaped bottle, stir magnetically, stir to make it all dissolve, then add HOSu (10.2g, 0.09mol), DCC (18.3g , 0.09mol), stirred overnight at room temperature, a large amount of white precipitate appeared, filtered, took the filtrate, added THF to make up the volume of 500ml, stirred at room temperature, and added D-H-His-OCH to the filtrate respectively 3 .2HCl ((23g, 0.096mol), NaHCO 3 (30g, 0.36mol), H 2 O150ml, stirred overnight at room temperature, TLC showed that the reaction was complete, treated, evaporated THF to dryness, added ethyl acetate and water, stirred, separated layers, took the ethyl ester layer, extracted the water layer with ethyl ester once, poured it off, and combined the ethyl ester layers , the ethyl ester layer was washed twice with dilute hydrochloric acid aqueous solution to remove excess H-His-OCH 3 , washed 3 times with...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com